 | |
| Combination of | |
|---|---|
| Olanzapine | Atypical antipsychotic |
| Fluoxetine | Selective serotonin reuptake inhibitor |
| Clinical data | |
| Trade names | Symbyax, Cinol Forte, Olapin Forte, others |
| AHFS/ Drugs.com | Professional Drug Facts |
| License data | |
|
Pregnancy category |
|
|
Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| KEGG |
|
| | |
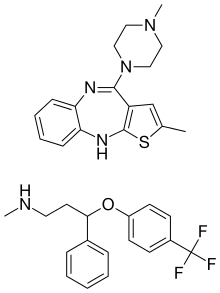
Olanzapine/fluoxetine (trade name Symbyax, created by Eli Lilly and Company) is a fixed-dose combination medication containing olanzapine (Zyprexa), an atypical antipsychotic, and fluoxetine (Prozac), a selective serotonin reuptake inhibitor (SSRI). Olanzapine/fluoxetine is primarily used to treat the depressive episodes of bipolar I disorder [2] as well as treatment-resistant depression. [1] [3]
Medical uses
Olanzapine/fluoxetine was approved by the U.S. Food and Drug Administration (FDA) to treat the depressive episodes of bipolar I disorder in 2003. [1] In 2009, it was granted approval for the treatment of treatment-resistant depression. [4]
Olanzapine/fluoxetine, or other antidepressant/ antipsychotic combinations, are sometimes prescribed off-label for anxiety disorders, [5] eating disorders, [6] obsessive–compulsive disorder (OCD), [7] and posttraumatic stress disorder (PTSD). [8]
Side effects
Possible side effects of olanzapine/fluoxetine include all those of the two component drugs: olanzapine (side effects) and fluoxetine (side effects). Common side effects include suicidal thoughts, increased appetite, weight gain, drowsiness, fatigue, dry mouth, swelling, tremor, blurred vision, and difficulty concentrating. [1]
Olanzapine/fluoxetine could produce a severe allergic reaction and should not be used if the patient has previously experienced an allergic reaction to either fluoxetine or olanzapine. [9]
Olanzapine is correlated with an increase in blood sugar. Patients with diabetes, or those at risk for developing it, require careful monitoring. [9]
In rare cases, olanzapine/fluoxetine may cause neuroleptic malignant syndrome. [1]
Like other SSRIs, olanzapine/fluoxetine carries a boxed warning stating that it could increase the risk of suicidal thoughts and behaviors in patients aged 24 and under. The warning also states that olanzapine/fluoxetine may increase the risk of death in elderly patients with dementia-related psychosis. [1]
See also
- Amitriptyline/perphenazine
- Aripiprazole/sertraline
- " California rocket fuel"
- Flupentixol/melitracen
- Tranylcypromine/trifluoperazine
References
- ^ a b c d e f "Symbyax- olanzapine and fluoxetine hydrochloride capsule". DailyMed. 21 April 2020. Retrieved 30 September 2020.
- ^ "Tratamento medicamentoso dos transtornos bipolares - Transtornos psiquiátricos". Manuais MSD edição para profissionais (in Brazilian Portuguese). Retrieved 2022-10-02.
- ^ Benazzi F, Berk M, Frye MA, Wang W, Barraco A, Tohen M (October 2009). "Olanzapine/fluoxetine combination for the treatment of mixed depression in bipolar I disorder: a post hoc analysis". The Journal of Clinical Psychiatry. 70 (10): 1424–1431. doi: 10.4088/JCP.08m04772gre. PMID 19906346.
- ^ Grohol, J. "FDA Approves Symbyax for Treatment Resistant Depression". Psych Central Blog. Archived from the original on 2017-12-26. Retrieved 2010-07-17.
- ^ McIntyre R, Katzman M (2003). "The role of atypical antipsychotics in bipolar depression and anxiety disorders". Bipolar Disorders. 5 (Suppl 2): 20–35. doi: 10.1111/j.1399-2406.2003.00061.x. PMID 14700010.
- ^ Pederson KJ, Roerig JL, Mitchell JE (October 2003). "Towards the pharmacotherapy of eating disorders". Expert Opinion on Pharmacotherapy. 4 (10): 1659–1678. doi: 10.1517/14656566.4.10.1659. PMID 14521477. S2CID 38506292.
- ^ Koran LM, Ringold AL, Elliott MA (July 2000). "Olanzapine augmentation for treatment-resistant obsessive-compulsive disorder". The Journal of Clinical Psychiatry. 61 (7): 514–517. doi: 10.4088/JCP.v61n0709. PMID 10937610.
- ^ Stein MB, Kline NA, Matloff JL (October 2002). "Adjunctive olanzapine for SSRI-resistant combat-related PTSD: a double-blind, placebo-controlled study". The American Journal of Psychiatry. 159 (10): 1777–1779. doi: 10.1176/appi.ajp.159.10.1777. PMID 12359687.
- ^ a b "Symbyax". Drugs.com.
External links
- "Fluoxetine hydrochloride mixture with Olanzapine". Drug Information Portal. U.S. National Library of Medicine.
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| Antidepressants |
|
|---|---|
| Others | |
- CS1 Brazilian Portuguese-language sources (pt-br)
- Articles with short description
- Short description matches Wikidata
- Articles with changed KEGG identifier
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without InChI source
- Articles without UNII source
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Drugs that are a combination of chemicals
 | |
| Combination of | |
|---|---|
| Olanzapine | Atypical antipsychotic |
| Fluoxetine | Selective serotonin reuptake inhibitor |
| Clinical data | |
| Trade names | Symbyax, Cinol Forte, Olapin Forte, others |
| AHFS/ Drugs.com | Professional Drug Facts |
| License data | |
|
Pregnancy category |
|
|
Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| KEGG |
|
| | |
Olanzapine/fluoxetine (trade name Symbyax, created by Eli Lilly and Company) is a fixed-dose combination medication containing olanzapine (Zyprexa), an atypical antipsychotic, and fluoxetine (Prozac), a selective serotonin reuptake inhibitor (SSRI). Olanzapine/fluoxetine is primarily used to treat the depressive episodes of bipolar I disorder [2] as well as treatment-resistant depression. [1] [3]
Medical uses
Olanzapine/fluoxetine was approved by the U.S. Food and Drug Administration (FDA) to treat the depressive episodes of bipolar I disorder in 2003. [1] In 2009, it was granted approval for the treatment of treatment-resistant depression. [4]
Olanzapine/fluoxetine, or other antidepressant/ antipsychotic combinations, are sometimes prescribed off-label for anxiety disorders, [5] eating disorders, [6] obsessive–compulsive disorder (OCD), [7] and posttraumatic stress disorder (PTSD). [8]
Side effects
Possible side effects of olanzapine/fluoxetine include all those of the two component drugs: olanzapine (side effects) and fluoxetine (side effects). Common side effects include suicidal thoughts, increased appetite, weight gain, drowsiness, fatigue, dry mouth, swelling, tremor, blurred vision, and difficulty concentrating. [1]
Olanzapine/fluoxetine could produce a severe allergic reaction and should not be used if the patient has previously experienced an allergic reaction to either fluoxetine or olanzapine. [9]
Olanzapine is correlated with an increase in blood sugar. Patients with diabetes, or those at risk for developing it, require careful monitoring. [9]
In rare cases, olanzapine/fluoxetine may cause neuroleptic malignant syndrome. [1]
Like other SSRIs, olanzapine/fluoxetine carries a boxed warning stating that it could increase the risk of suicidal thoughts and behaviors in patients aged 24 and under. The warning also states that olanzapine/fluoxetine may increase the risk of death in elderly patients with dementia-related psychosis. [1]
See also
- Amitriptyline/perphenazine
- Aripiprazole/sertraline
- " California rocket fuel"
- Flupentixol/melitracen
- Tranylcypromine/trifluoperazine
References
- ^ a b c d e f "Symbyax- olanzapine and fluoxetine hydrochloride capsule". DailyMed. 21 April 2020. Retrieved 30 September 2020.
- ^ "Tratamento medicamentoso dos transtornos bipolares - Transtornos psiquiátricos". Manuais MSD edição para profissionais (in Brazilian Portuguese). Retrieved 2022-10-02.
- ^ Benazzi F, Berk M, Frye MA, Wang W, Barraco A, Tohen M (October 2009). "Olanzapine/fluoxetine combination for the treatment of mixed depression in bipolar I disorder: a post hoc analysis". The Journal of Clinical Psychiatry. 70 (10): 1424–1431. doi: 10.4088/JCP.08m04772gre. PMID 19906346.
- ^ Grohol, J. "FDA Approves Symbyax for Treatment Resistant Depression". Psych Central Blog. Archived from the original on 2017-12-26. Retrieved 2010-07-17.
- ^ McIntyre R, Katzman M (2003). "The role of atypical antipsychotics in bipolar depression and anxiety disorders". Bipolar Disorders. 5 (Suppl 2): 20–35. doi: 10.1111/j.1399-2406.2003.00061.x. PMID 14700010.
- ^ Pederson KJ, Roerig JL, Mitchell JE (October 2003). "Towards the pharmacotherapy of eating disorders". Expert Opinion on Pharmacotherapy. 4 (10): 1659–1678. doi: 10.1517/14656566.4.10.1659. PMID 14521477. S2CID 38506292.
- ^ Koran LM, Ringold AL, Elliott MA (July 2000). "Olanzapine augmentation for treatment-resistant obsessive-compulsive disorder". The Journal of Clinical Psychiatry. 61 (7): 514–517. doi: 10.4088/JCP.v61n0709. PMID 10937610.
- ^ Stein MB, Kline NA, Matloff JL (October 2002). "Adjunctive olanzapine for SSRI-resistant combat-related PTSD: a double-blind, placebo-controlled study". The American Journal of Psychiatry. 159 (10): 1777–1779. doi: 10.1176/appi.ajp.159.10.1777. PMID 12359687.
- ^ a b "Symbyax". Drugs.com.
External links
- "Fluoxetine hydrochloride mixture with Olanzapine". Drug Information Portal. U.S. National Library of Medicine.
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
Anxiolytics (
N05B) | |
|---|---|
| 5-HT1ARTooltip 5-HT1A receptor agonists | |
| GABAARTooltip GABAA receptor PAMsTooltip positive allosteric modulators |
|
| Hypnotics | |
|
Gabapentinoids ( α2δ VDCC blockers) | |
| Antidepressants |
|
| Antipsychotics | |
|
Sympatholytics ( Antiadrenergics) |
|
| Others | |
| |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Antidepressants |
|
|---|---|
| Others | |
- CS1 Brazilian Portuguese-language sources (pt-br)
- Articles with short description
- Short description matches Wikidata
- Articles with changed KEGG identifier
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without InChI source
- Articles without UNII source
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Drugs that are a combination of chemicals