
| |
| Names | |
|---|---|
|
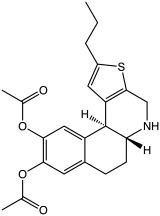
Preferred IUPAC name
(5aR,11bS)-2-Propyl-4,5,5a,6,7,11b-hexahydrobenzo[f]thieno[2,3-c]quinoline-9,10-diol | |
| Identifiers | |
3D model (
JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem
CID
|
|
| UNII | |
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C18H21NO2S | |
| Molar mass | 315.429 g/mol |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
A-86929 is a synthetic compound that acts as a selective dopamine receptor D1 agonist. [1] [2] It was developed as a possible treatment for Parkinson's disease, [3] as well as for other applications such as treatment of cocaine addiction, [4] [5] but while it had reasonable efficacy in humans it also caused dyskinesias and has not been continued. [6] [7] It has mainly been used as its di acetate ester prodrug adrogolide (ABT-431), which has better bioavailability. [8] [9]
References
- ^ Michaelides MR, Hong Y, DiDomenico S, Asin KE, Britton DR, Lin CW, Williams M, Shiosaki K (September 1995). "(5aR,11bS)-4,5,5a,6,7,11b-hexahydro-2-propyl-3-thia-5-azacyclopent-1- ena[c]-phenanthrene-9,10-diol (A-86929): a potent and selective dopamine D1 agonist that maintains behavioral efficacy following repeated administration and characterization of its diacetyl prodrug (ABT-431)". Journal of Medicinal Chemistry. 38 (18): 3445–7. doi: 10.1021/jm00018a002. PMID 7658429.
- ^ Ehrlich PP, Ralston JW, Michaelides MR (May 1997). "An Efficient Enantioselective Synthesis of the D1 Agonist (5aR,11bS)-4,5,5a,6,7,11b-Hexahydro-2-propyl-3-thia- 5-azacyclopenta[c]phenanthrene-9,10-diol (A-86929)". The Journal of Organic Chemistry. 62 (9): 2782–2785. doi: 10.1021/jo970066l. PMID 11671640.
- ^ Rascol O, Blin O, Thalamas C, Descombes S, Soubrouillard C, Azulay P, Fabre N, Viallet F, Lafnitzegger K, Wright S, Carter JH, Nutt JG (June 1999). "ABT-431, a D1 receptor agonist prodrug, has efficacy in Parkinson's disease". Annals of Neurology. 45 (6): 736–41. doi: 10.1002/1531-8249(199906)45:6<736::AID-ANA7>3.0.CO;2-F. PMID 10360765. S2CID 26090362.
- ^ Haney M, Collins ED, Ward AS, Foltin RW, Fischman MW (March 1999). "Effect of a selective dopamine D1 agonist (ABT-431) on smoked cocaine self-administration in humans". Psychopharmacology. 143 (1): 102–10. doi: 10.1007/s002130050925. PMID 10227086. S2CID 24283309.
- ^ Gorelick DA, Gardner EL, Xi ZX (2004). "Agents in development for the management of cocaine abuse". Drugs. 64 (14): 1547–73. doi: 10.2165/00003495-200464140-00004. PMID 15233592. S2CID 5421657.
- ^ Rascol O, Nutt JG, Blin O, Goetz CG, Trugman JM, Soubrouillard C, Carter JH, Currie LJ, Fabre N, Thalamas C, Giardina WW, Wright S (February 2001). "Induction by dopamine D1 receptor agonist ABT-431 of dyskinesia similar to levodopa in patients with Parkinson disease". Archives of Neurology. 58 (2): 249–54. doi: 10.1001/archneur.58.2.249. PMID 11176963.
- ^ Zhang J, Xiong B, Zhen X, Zhang A (March 2009). "Dopamine D1 receptor ligands: where are we now and where are we going". Medicinal Research Reviews. 29 (2): 272–94. doi: 10.1002/med.20130. PMID 18642350. S2CID 25334596.
- ^ Shiosaki K, Jenner P, Asin KE, Britton DR, Lin CW, Michaelides M, Smith L, Bianchi B, Didomenico S, Hodges L, Hong Y, Mahan L, Mikusa J, Miller T, Nikkel A, Stashko M, Witte D, Williams M (January 1996). "ABT-431: the diacetyl prodrug of A-86929, a potent and selective dopamine D1 receptor agonist: in vitro characterization and effects in animal models of Parkinson's disease". The Journal of Pharmacology and Experimental Therapeutics. 276 (1): 150–60. PMID 8558425.
- ^ Giardina WJ, Williams M (2001). "Adrogolide HCl (ABT-431; DAS-431), a prodrug of the dopamine D1 receptor agonist, A-86929: preclinical pharmacology and clinical data". CNS Drug Reviews. 7 (3): 305–16. doi: 10.1111/j.1527-3458.2001.tb00201.x. PMC 6741696. PMID 11607045.

| |
| Names | |
|---|---|
|
Preferred IUPAC name
(5aR,11bS)-2-Propyl-4,5,5a,6,7,11b-hexahydrobenzo[f]thieno[2,3-c]quinoline-9,10-diol | |
| Identifiers | |
3D model (
JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem
CID
|
|
| UNII | |
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C18H21NO2S | |
| Molar mass | 315.429 g/mol |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
A-86929 is a synthetic compound that acts as a selective dopamine receptor D1 agonist. [1] [2] It was developed as a possible treatment for Parkinson's disease, [3] as well as for other applications such as treatment of cocaine addiction, [4] [5] but while it had reasonable efficacy in humans it also caused dyskinesias and has not been continued. [6] [7] It has mainly been used as its di acetate ester prodrug adrogolide (ABT-431), which has better bioavailability. [8] [9]

References
- ^ Michaelides MR, Hong Y, DiDomenico S, Asin KE, Britton DR, Lin CW, Williams M, Shiosaki K (September 1995). "(5aR,11bS)-4,5,5a,6,7,11b-hexahydro-2-propyl-3-thia-5-azacyclopent-1- ena[c]-phenanthrene-9,10-diol (A-86929): a potent and selective dopamine D1 agonist that maintains behavioral efficacy following repeated administration and characterization of its diacetyl prodrug (ABT-431)". Journal of Medicinal Chemistry. 38 (18): 3445–7. doi: 10.1021/jm00018a002. PMID 7658429.
- ^ Ehrlich PP, Ralston JW, Michaelides MR (May 1997). "An Efficient Enantioselective Synthesis of the D1 Agonist (5aR,11bS)-4,5,5a,6,7,11b-Hexahydro-2-propyl-3-thia- 5-azacyclopenta[c]phenanthrene-9,10-diol (A-86929)". The Journal of Organic Chemistry. 62 (9): 2782–2785. doi: 10.1021/jo970066l. PMID 11671640.
- ^ Rascol O, Blin O, Thalamas C, Descombes S, Soubrouillard C, Azulay P, Fabre N, Viallet F, Lafnitzegger K, Wright S, Carter JH, Nutt JG (June 1999). "ABT-431, a D1 receptor agonist prodrug, has efficacy in Parkinson's disease". Annals of Neurology. 45 (6): 736–41. doi: 10.1002/1531-8249(199906)45:6<736::AID-ANA7>3.0.CO;2-F. PMID 10360765. S2CID 26090362.
- ^ Haney M, Collins ED, Ward AS, Foltin RW, Fischman MW (March 1999). "Effect of a selective dopamine D1 agonist (ABT-431) on smoked cocaine self-administration in humans". Psychopharmacology. 143 (1): 102–10. doi: 10.1007/s002130050925. PMID 10227086. S2CID 24283309.
- ^ Gorelick DA, Gardner EL, Xi ZX (2004). "Agents in development for the management of cocaine abuse". Drugs. 64 (14): 1547–73. doi: 10.2165/00003495-200464140-00004. PMID 15233592. S2CID 5421657.
- ^ Rascol O, Nutt JG, Blin O, Goetz CG, Trugman JM, Soubrouillard C, Carter JH, Currie LJ, Fabre N, Thalamas C, Giardina WW, Wright S (February 2001). "Induction by dopamine D1 receptor agonist ABT-431 of dyskinesia similar to levodopa in patients with Parkinson disease". Archives of Neurology. 58 (2): 249–54. doi: 10.1001/archneur.58.2.249. PMID 11176963.
- ^ Zhang J, Xiong B, Zhen X, Zhang A (March 2009). "Dopamine D1 receptor ligands: where are we now and where are we going". Medicinal Research Reviews. 29 (2): 272–94. doi: 10.1002/med.20130. PMID 18642350. S2CID 25334596.
- ^ Shiosaki K, Jenner P, Asin KE, Britton DR, Lin CW, Michaelides M, Smith L, Bianchi B, Didomenico S, Hodges L, Hong Y, Mahan L, Mikusa J, Miller T, Nikkel A, Stashko M, Witte D, Williams M (January 1996). "ABT-431: the diacetyl prodrug of A-86929, a potent and selective dopamine D1 receptor agonist: in vitro characterization and effects in animal models of Parkinson's disease". The Journal of Pharmacology and Experimental Therapeutics. 276 (1): 150–60. PMID 8558425.
- ^ Giardina WJ, Williams M (2001). "Adrogolide HCl (ABT-431; DAS-431), a prodrug of the dopamine D1 receptor agonist, A-86929: preclinical pharmacology and clinical data". CNS Drug Reviews. 7 (3): 305–16. doi: 10.1111/j.1527-3458.2001.tb00201.x. PMC 6741696. PMID 11607045.