 | |
| Clinical data | |
|---|---|
| Other names | HT-11 |
| AHFS/ Drugs.com | International Drug Names |
|
Routes of administration | Oral |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard ( EPA) | |
| ECHA InfoCard | 100.020.948 |
| Chemical and physical data | |
| Formula | C20H24ClNO |
| Molar mass | 329.87 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
| | |
Cloperastine ( INN) or cloperastin, in the forms of cloperastine hydrochloride ( JAN) (brand names Hustazol, Nitossil, Seki) and cloperastine fendizoate, is an antitussive and antihistamine that is marketed as a cough suppressant in Japan, Hong Kong, and in some European countries. [1] [2] [3] It was first introduced in 1972 in Japan, and then in Italy in 1981. [4]
Side effects
Adverse effects may include sedation, drowsiness, heartburn, and thickening of bronchial secretions. [5]
Pharmacology
The precise mechanism of action of cloperastine is not fully clear, but several different biological activities have been identified for the drug, of which include: ligand of the σ1 receptor (Ki = 20 nM) (likely an agonist), [6] GIRK channel blocker (described as "potent"), [7] [8] [9] [10] antihistamine (Ki = 3.8 nM for the H1 receptor), [3] [6] and anticholinergic. [3] [11] It is thought that the latter two properties contribute to side effects, such as sedation and somnolence, while the former two may be involved in or responsible for the antitussive efficacy of cloperastine. [6] [7]
Synthesis
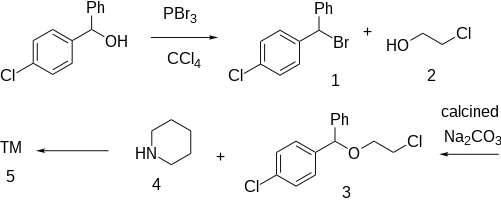
The halogenation of 4-Chlorobenzhydrol [119-56-2] (1) with phosphorus tribromide in tetrachloromethane gives 1-(Bromophenylmethyl)-4-chlorobenzene [948-54-9] (2). Treatment with ethylenechlorohydrin ( 2-Chloroethanol) [107-07-3] (3) gives 1-(4-Chlorobenzhydryl)oxy-2-chloroethane [5321-46-0] (4). Reaction with piperidine (5) completes the synthesis of Cloperastine (6).
See also
- Cough syrup
- Noscapine
- Codeine; Pholcodine
- Dextromethorphan; Dimemorfan
- Racemorphan; Dextrorphan; Levorphanol
- Butamirate
- Pentoxyverine
- Tipepidine
- Levocloperastine
References
- ^ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 301–. ISBN 978-1-4757-2085-3.
- ^ Swiss Pharmaceutial Society, ed. (January 2000). Index Nominum 2000: International Drug Directory. Taylor & Francis. pp. 261–. ISBN 978-3-88763-075-1.
- ^ a b c Catania MA, Cuzzocrea S (2011). "Pharmacological and clinical overview of cloperastine in treatment of cough". Therapeutics and Clinical Risk Management. 7: 83–92. doi: 10.2147/TCRM.S16643. PMC 3061847. PMID 21445282.
- ^ William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia. Elsevier. pp. 1103–. ISBN 978-0-8155-1856-3.
- ^ Schlesser JL (1991). Drugs Available Abroad, 1st Edition. Derwent Publications Ltd. p. 29. ISBN 0-8103-7177-4.
- ^ a b c Gregori-Puigjané E, Setola V, Hert J, Crews BA, Irwin JJ, Lounkine E, et al. (July 2012). "Identifying mechanism-of-action targets for drugs and probes". Proceedings of the National Academy of Sciences of the United States of America. 109 (28): 11178–83. Bibcode: 2012PNAS..10911178G. doi: 10.1073/pnas.1204524109. PMC 3396511. PMID 22711801.
- ^ a b Chung KF, Widdicombe J (30 September 2008). Pharmacology and Therapeutics of Cough. Springer Science & Business Media. pp. 230–. ISBN 9783540798422.
- ^ Soeda F, Fujieda Y, Kinoshita M, Shirasaki T, Takahama K (May 2016). "Centrally acting non-narcotic antitussives prevent hyperactivity in mice: Involvement of GIRK channels". Pharmacology, Biochemistry, and Behavior. 144: 26–32. doi: 10.1016/j.pbb.2016.02.006. ISBN 978-3-540-79842-2. OCLC 612742272. PMID 26892760. S2CID 30118634.
- ^ Yamamoto G, Soeda F, Shirasaki T, Takahama K (April 2011). "[Is the GIRK channel a possible target in the development of a novel therapeutic drug of urinary disturbance?]". Yakugaku Zasshi. 131 (4): 523–32. doi: 10.1248/yakushi.131.523. PMID 21467791.
- ^ Kawaura K, Honda S, Soeda F, Shirasaki T, Takahama K (May 2010). "[Novel antidepressant-like action of drugs possessing GIRK channel blocking action in rats]". Yakugaku Zasshi. 130 (5): 699–705. doi: 10.1248/yakushi.130.699. PMID 20460867.
- ^ Korolkovas A (16 August 1988). Essentials of Medicinal Chemistry. Wiley. ISBN 978-0-471-88356-2.
- ^ Arnold H, Brock N, Kuhas E, Lorenz D (March 1954). "[Effect of antihistaminic substances. I. Chemical constitution and pharmacological effect of the basic benzhydrylethers]". Arzneimittel-Forschung. 4 (3): 189–194. PMID 13159698.
- ^ Anon., GB 1179945 (1970 to Yoshitomi Pharmaceutical).
- ^ Anon., GB 670622 (1952 to Parke Davis & Co).
- ^ Laura Puricelli, EP 0894794 (1999 to AESCULAPIUS FARMACEUTICI S.r.l.).
- ^ 陶文潘, 潘文驰, 潘兴长, 罗泳萍, 樊希祥, CN 104327014A (2015 to 重庆市恒安化工有限公司).
 | |
| Clinical data | |
|---|---|
| Other names | HT-11 |
| AHFS/ Drugs.com | International Drug Names |
|
Routes of administration | Oral |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard ( EPA) | |
| ECHA InfoCard | 100.020.948 |
| Chemical and physical data | |
| Formula | C20H24ClNO |
| Molar mass | 329.87 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
| | |
Cloperastine ( INN) or cloperastin, in the forms of cloperastine hydrochloride ( JAN) (brand names Hustazol, Nitossil, Seki) and cloperastine fendizoate, is an antitussive and antihistamine that is marketed as a cough suppressant in Japan, Hong Kong, and in some European countries. [1] [2] [3] It was first introduced in 1972 in Japan, and then in Italy in 1981. [4]
Side effects
Adverse effects may include sedation, drowsiness, heartburn, and thickening of bronchial secretions. [5]
Pharmacology
The precise mechanism of action of cloperastine is not fully clear, but several different biological activities have been identified for the drug, of which include: ligand of the σ1 receptor (Ki = 20 nM) (likely an agonist), [6] GIRK channel blocker (described as "potent"), [7] [8] [9] [10] antihistamine (Ki = 3.8 nM for the H1 receptor), [3] [6] and anticholinergic. [3] [11] It is thought that the latter two properties contribute to side effects, such as sedation and somnolence, while the former two may be involved in or responsible for the antitussive efficacy of cloperastine. [6] [7]
Synthesis

The halogenation of 4-Chlorobenzhydrol [119-56-2] (1) with phosphorus tribromide in tetrachloromethane gives 1-(Bromophenylmethyl)-4-chlorobenzene [948-54-9] (2). Treatment with ethylenechlorohydrin ( 2-Chloroethanol) [107-07-3] (3) gives 1-(4-Chlorobenzhydryl)oxy-2-chloroethane [5321-46-0] (4). Reaction with piperidine (5) completes the synthesis of Cloperastine (6).
See also
- Cough syrup
- Noscapine
- Codeine; Pholcodine
- Dextromethorphan; Dimemorfan
- Racemorphan; Dextrorphan; Levorphanol
- Butamirate
- Pentoxyverine
- Tipepidine
- Levocloperastine
References
- ^ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 301–. ISBN 978-1-4757-2085-3.
- ^ Swiss Pharmaceutial Society, ed. (January 2000). Index Nominum 2000: International Drug Directory. Taylor & Francis. pp. 261–. ISBN 978-3-88763-075-1.
- ^ a b c Catania MA, Cuzzocrea S (2011). "Pharmacological and clinical overview of cloperastine in treatment of cough". Therapeutics and Clinical Risk Management. 7: 83–92. doi: 10.2147/TCRM.S16643. PMC 3061847. PMID 21445282.
- ^ William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia. Elsevier. pp. 1103–. ISBN 978-0-8155-1856-3.
- ^ Schlesser JL (1991). Drugs Available Abroad, 1st Edition. Derwent Publications Ltd. p. 29. ISBN 0-8103-7177-4.
- ^ a b c Gregori-Puigjané E, Setola V, Hert J, Crews BA, Irwin JJ, Lounkine E, et al. (July 2012). "Identifying mechanism-of-action targets for drugs and probes". Proceedings of the National Academy of Sciences of the United States of America. 109 (28): 11178–83. Bibcode: 2012PNAS..10911178G. doi: 10.1073/pnas.1204524109. PMC 3396511. PMID 22711801.
- ^ a b Chung KF, Widdicombe J (30 September 2008). Pharmacology and Therapeutics of Cough. Springer Science & Business Media. pp. 230–. ISBN 9783540798422.
- ^ Soeda F, Fujieda Y, Kinoshita M, Shirasaki T, Takahama K (May 2016). "Centrally acting non-narcotic antitussives prevent hyperactivity in mice: Involvement of GIRK channels". Pharmacology, Biochemistry, and Behavior. 144: 26–32. doi: 10.1016/j.pbb.2016.02.006. ISBN 978-3-540-79842-2. OCLC 612742272. PMID 26892760. S2CID 30118634.
- ^ Yamamoto G, Soeda F, Shirasaki T, Takahama K (April 2011). "[Is the GIRK channel a possible target in the development of a novel therapeutic drug of urinary disturbance?]". Yakugaku Zasshi. 131 (4): 523–32. doi: 10.1248/yakushi.131.523. PMID 21467791.
- ^ Kawaura K, Honda S, Soeda F, Shirasaki T, Takahama K (May 2010). "[Novel antidepressant-like action of drugs possessing GIRK channel blocking action in rats]". Yakugaku Zasshi. 130 (5): 699–705. doi: 10.1248/yakushi.130.699. PMID 20460867.
- ^ Korolkovas A (16 August 1988). Essentials of Medicinal Chemistry. Wiley. ISBN 978-0-471-88356-2.
- ^ Arnold H, Brock N, Kuhas E, Lorenz D (March 1954). "[Effect of antihistaminic substances. I. Chemical constitution and pharmacological effect of the basic benzhydrylethers]". Arzneimittel-Forschung. 4 (3): 189–194. PMID 13159698.
- ^ Anon., GB 1179945 (1970 to Yoshitomi Pharmaceutical).
- ^ Anon., GB 670622 (1952 to Parke Davis & Co).
- ^ Laura Puricelli, EP 0894794 (1999 to AESCULAPIUS FARMACEUTICI S.r.l.).
- ^ 陶文潘, 潘文驰, 潘兴长, 罗泳萍, 樊希祥, CN 104327014A (2015 to 重庆市恒安化工有限公司).