 | |
| Clinical data | |
|---|---|
| Trade names | Tarlige |
| Other names | DS-5565 |
|
Routes of administration | By mouth |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
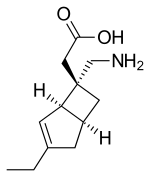
| Formula | C12H19NO2 |
| Molar mass | 209.289 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
Mirogabalin (brand name Tarlige; developmental code name DS-5565) is a gabapentinoid medication developed by Daiichi Sankyo. Gabapentin and pregabalin are also members of this class. As a gabapentinoid, mirogabalin binds to the α2δ subunit of voltage-gated calcium channel ( 1 and 2), but with significantly higher potency than pregabalin. It has shown promising results in Phase II clinical trials for the treatment of diabetic peripheral neuropathic pain. [1] [2]
Phase III trial results:
- Effective: for post-herpetic neuralgia (trial: NEUCOURSE)
- Ineffective: for fibromyalgia (trial: ALDAY) [3]
- Effective: for diabetic peripheral neuropathic pain (trial: REDUCER) [4]
In Japan, the company submitted a marketing application for treatment of peripheral neuropathic pain. [5] The medication was approved for neuropathic pain and postherpetic neuralgia in Japan in January 2019. [6]
References
- ^ Vinik A, Rosenstock J, Sharma U, Feins K, Hsu C, Merante D (December 2014). "Efficacy and safety of mirogabalin (DS-5565) for the treatment of diabetic peripheral neuropathic pain: a randomized, double-blind, placebo- and active comparator-controlled, adaptive proof-of-concept phase 2 study". Diabetes Care. 37 (12): 3253–61. doi: 10.2337/dc14-1044. PMID 25231896.
- ^ Vinik A, Sharma U, Feins K, Hsu C, Merante D (2014). "DS-5565 for the Treatment Of Diabetic Peripheral Neuropathic Pain: Randomized, Double-Blind, Placebo- And Active Comparator-Controlled Phase II Study (S20.004)". Neurology. 82 (10): S20.004.
- ^ "Daiichi Sankyo Announces Top-line Results from Phase 3 Global Clinical Development Program Evaluating Mirogabalin in Pain Syndromes". Daiichi Sankyo. 30 June 2017.
- ^ "Daiichi Sankyo Announces Positive Top-line Results from Phase 3 Clinical Trial Evaluating Mirogabalin in Diabetic Peripheral Neuropathic Pain". Daiichi Sankyo. 31 August 2017.
- ^ "Daiichi Sankyo Submits Marketing Application for Mirogabalin in Japan". Daiichi Sankyo. 15 February 2018.
- ^ "Mirogabalin - Daiichi Sankyo Company - AdisInsight".
External links
- "Mirogabalin". Drug Information Portal. U.S. National Library of Medicine.
 | |
| Clinical data | |
|---|---|
| Trade names | Tarlige |
| Other names | DS-5565 |
|
Routes of administration | By mouth |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C12H19NO2 |
| Molar mass | 209.289 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
Mirogabalin (brand name Tarlige; developmental code name DS-5565) is a gabapentinoid medication developed by Daiichi Sankyo. Gabapentin and pregabalin are also members of this class. As a gabapentinoid, mirogabalin binds to the α2δ subunit of voltage-gated calcium channel ( 1 and 2), but with significantly higher potency than pregabalin. It has shown promising results in Phase II clinical trials for the treatment of diabetic peripheral neuropathic pain. [1] [2]
Phase III trial results:
- Effective: for post-herpetic neuralgia (trial: NEUCOURSE)
- Ineffective: for fibromyalgia (trial: ALDAY) [3]
- Effective: for diabetic peripheral neuropathic pain (trial: REDUCER) [4]
In Japan, the company submitted a marketing application for treatment of peripheral neuropathic pain. [5] The medication was approved for neuropathic pain and postherpetic neuralgia in Japan in January 2019. [6]
References
- ^ Vinik A, Rosenstock J, Sharma U, Feins K, Hsu C, Merante D (December 2014). "Efficacy and safety of mirogabalin (DS-5565) for the treatment of diabetic peripheral neuropathic pain: a randomized, double-blind, placebo- and active comparator-controlled, adaptive proof-of-concept phase 2 study". Diabetes Care. 37 (12): 3253–61. doi: 10.2337/dc14-1044. PMID 25231896.
- ^ Vinik A, Sharma U, Feins K, Hsu C, Merante D (2014). "DS-5565 for the Treatment Of Diabetic Peripheral Neuropathic Pain: Randomized, Double-Blind, Placebo- And Active Comparator-Controlled Phase II Study (S20.004)". Neurology. 82 (10): S20.004.
- ^ "Daiichi Sankyo Announces Top-line Results from Phase 3 Global Clinical Development Program Evaluating Mirogabalin in Pain Syndromes". Daiichi Sankyo. 30 June 2017.
- ^ "Daiichi Sankyo Announces Positive Top-line Results from Phase 3 Clinical Trial Evaluating Mirogabalin in Diabetic Peripheral Neuropathic Pain". Daiichi Sankyo. 31 August 2017.
- ^ "Daiichi Sankyo Submits Marketing Application for Mirogabalin in Japan". Daiichi Sankyo. 15 February 2018.
- ^ "Mirogabalin - Daiichi Sankyo Company - AdisInsight".
External links
- "Mirogabalin". Drug Information Portal. U.S. National Library of Medicine.