 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard ( EPA) | |
| ECHA InfoCard | 100.120.272 |
| Chemical and physical data | |
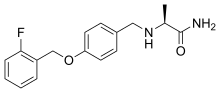
| Formula | C17H19FN2O2 |
| Molar mass | 302.349 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
Ralfinamide ( INN) (code names NW-1029, FCE-26742A, PNU-0154339E) [1] is a multimodal drug which is under investigation by Newron Pharmaceuticals for the treatment of neuropathic pain and other pain conditions such as post-operative dental pain. [2] [3] [4] [5]
It has a relatively complex pharmacology, acting as a mixed voltage-gated sodium channel blocker (including Nav1.7), [2] [3] N-type calcium channel blocker, [2] [3] noncompetitive NMDA receptor antagonist, [6] and monoamine oxidase B inhibitor. [7] [8]
It has thus far progressed as far as phase IIb/ phase III clinical trials. [5] [9] In 2010 it failed a phase II trial for lower back pain. [10] Encouraging Phase II results have been announced for neuropathic pain. [11]
See also
- List of investigational analgesics
- Safinamide, different fluorine position
- Evenamide, structurally-related antipsychotic in development
- Lacosamide, used for partial-onset seizures and diabetic neuropathic pain
- Ziconotide, FDA approved peptide for chronic neuropathic pain
References
- ^ Action A, ed. (22 July 2013). "Chapter 8: Therapies and Treatments". Pain: New Insights for the Healthcare Professional (2013 ed.). ScholarlyEditions. pp. 506–. ISBN 978-1-4816-6118-8.
- ^ a b c Gilron I (21 June 2012). "Drug Discovery for Neuropathic Pain". In Simpson DM, McArthur JC, Dworkin RH (eds.). Neuropathic Pain: Mechanisms, Diagnosis and Treatment. Oxford University Press. pp. 40–. ISBN 978-0-19-539470-2.
- ^ a b c Rodger IW, Lacouture PG (14 October 2010). "Overview: Novel Targets for New Analgesics". In Sinatra RS, Jahr JS, Watkins-Pitchford JM (eds.). The Essence of Analgesia and Analgesics. Cambridge University Press. pp. 436–. ISBN 978-1-139-49198-3.
- ^ Termin A, Martinborough E, Wilson D (17 December 2008). "Recent Advances in Voltage-Gated Sodium Chanel Blockers: Therapeutic Potential as Drug Targets in the CNS". Annual Reports in Medicinal Chemistry. Academic Press. pp. 55–. ISBN 978-0-08-092187-7.
- ^ a b Liu Y, Qin N (26 January 2010). "Pharacological Modulation of Ion Channels for the Treatment of Chronic Pain". In Lu C, Li AP (eds.). Enzyme Inhibition in Drug Discovery and Development: The Good and the Bad. John Wiley & Sons. pp. 689–. ISBN 978-0-470-53894-4.
- ^ Colombo E, Curatolo L, Caccia C, Salvati P, Faravelli L (2007). "344 Ralfinamide Acts Through Nmda Receptor Complex: A Central Role for Chronic Pain Treatment". European Journal of Pain. 11 (S1): S152–S153. doi: 10.1016/j.ejpain.2007.03.359. ISSN 1090-3801. S2CID 58186567.
- ^ Di Stefano AF, Radicioni MM, Rusca A (May 2013). "Pressor response to oral tyramine and monoamine oxidase inhibition during treatment with ralfinamide (NW-1029)". Neurotoxicity Research. 23 (4): 315–326. doi: 10.1007/s12640-012-9344-5. PMID 22872464. S2CID 207442119.
- ^ Rang HP, Dale MM, Ritter JM, Flower RJ, Henderson G (14 April 2011). Rang & Dale's Pharmacology: with STUDENT CONSULT Online Access. Elsevier Health Sciences. pp. 2476–. ISBN 978-0-7020-4504-2.
- ^ Bowlby MR, Kaftan (9 December 2008). "Sodium Channel Blockers for the Treatment of Chronic Pain". In Gribkoff VK, Kaczmarek LK (eds.). Structure, Function and Modulation of Neuronal Voltage-Gated Ion Channels. John Wiley & Sons. pp. 377–. ISBN 978-0-470-42989-1.
- ^ "Newron reports SERENA trial top-line results for ralfinamide". Bloomberg. 6 May 2010. Archived from the original on 24 September 2015.
- ^ "Newron Announces Positive Results With Ralfinamide From Phase II Trial in Neuropathic Pain". PR Newswire. 26 October 2014. Archived from the original on 20 February 2015.
External links
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard ( EPA) | |
| ECHA InfoCard | 100.120.272 |
| Chemical and physical data | |
| Formula | C17H19FN2O2 |
| Molar mass | 302.349 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
Ralfinamide ( INN) (code names NW-1029, FCE-26742A, PNU-0154339E) [1] is a multimodal drug which is under investigation by Newron Pharmaceuticals for the treatment of neuropathic pain and other pain conditions such as post-operative dental pain. [2] [3] [4] [5]
It has a relatively complex pharmacology, acting as a mixed voltage-gated sodium channel blocker (including Nav1.7), [2] [3] N-type calcium channel blocker, [2] [3] noncompetitive NMDA receptor antagonist, [6] and monoamine oxidase B inhibitor. [7] [8]
It has thus far progressed as far as phase IIb/ phase III clinical trials. [5] [9] In 2010 it failed a phase II trial for lower back pain. [10] Encouraging Phase II results have been announced for neuropathic pain. [11]
See also
- List of investigational analgesics
- Safinamide, different fluorine position
- Evenamide, structurally-related antipsychotic in development
- Lacosamide, used for partial-onset seizures and diabetic neuropathic pain
- Ziconotide, FDA approved peptide for chronic neuropathic pain
References
- ^ Action A, ed. (22 July 2013). "Chapter 8: Therapies and Treatments". Pain: New Insights for the Healthcare Professional (2013 ed.). ScholarlyEditions. pp. 506–. ISBN 978-1-4816-6118-8.
- ^ a b c Gilron I (21 June 2012). "Drug Discovery for Neuropathic Pain". In Simpson DM, McArthur JC, Dworkin RH (eds.). Neuropathic Pain: Mechanisms, Diagnosis and Treatment. Oxford University Press. pp. 40–. ISBN 978-0-19-539470-2.
- ^ a b c Rodger IW, Lacouture PG (14 October 2010). "Overview: Novel Targets for New Analgesics". In Sinatra RS, Jahr JS, Watkins-Pitchford JM (eds.). The Essence of Analgesia and Analgesics. Cambridge University Press. pp. 436–. ISBN 978-1-139-49198-3.
- ^ Termin A, Martinborough E, Wilson D (17 December 2008). "Recent Advances in Voltage-Gated Sodium Chanel Blockers: Therapeutic Potential as Drug Targets in the CNS". Annual Reports in Medicinal Chemistry. Academic Press. pp. 55–. ISBN 978-0-08-092187-7.
- ^ a b Liu Y, Qin N (26 January 2010). "Pharacological Modulation of Ion Channels for the Treatment of Chronic Pain". In Lu C, Li AP (eds.). Enzyme Inhibition in Drug Discovery and Development: The Good and the Bad. John Wiley & Sons. pp. 689–. ISBN 978-0-470-53894-4.
- ^ Colombo E, Curatolo L, Caccia C, Salvati P, Faravelli L (2007). "344 Ralfinamide Acts Through Nmda Receptor Complex: A Central Role for Chronic Pain Treatment". European Journal of Pain. 11 (S1): S152–S153. doi: 10.1016/j.ejpain.2007.03.359. ISSN 1090-3801. S2CID 58186567.
- ^ Di Stefano AF, Radicioni MM, Rusca A (May 2013). "Pressor response to oral tyramine and monoamine oxidase inhibition during treatment with ralfinamide (NW-1029)". Neurotoxicity Research. 23 (4): 315–326. doi: 10.1007/s12640-012-9344-5. PMID 22872464. S2CID 207442119.
- ^ Rang HP, Dale MM, Ritter JM, Flower RJ, Henderson G (14 April 2011). Rang & Dale's Pharmacology: with STUDENT CONSULT Online Access. Elsevier Health Sciences. pp. 2476–. ISBN 978-0-7020-4504-2.
- ^ Bowlby MR, Kaftan (9 December 2008). "Sodium Channel Blockers for the Treatment of Chronic Pain". In Gribkoff VK, Kaczmarek LK (eds.). Structure, Function and Modulation of Neuronal Voltage-Gated Ion Channels. John Wiley & Sons. pp. 377–. ISBN 978-0-470-42989-1.
- ^ "Newron reports SERENA trial top-line results for ralfinamide". Bloomberg. 6 May 2010. Archived from the original on 24 September 2015.
- ^ "Newron Announces Positive Results With Ralfinamide From Phase II Trial in Neuropathic Pain". PR Newswire. 26 October 2014. Archived from the original on 20 February 2015.
External links