 | |
| Clinical data | |
|---|---|
| Other names | 9-Benzyl-2-methyl-2,3,4,9-tetrahydro-1H-gamma-carboline, Incidal, Omeril, Diazolin, Fabahistin, mebhydrolin napadisylate, mebhydroline 1,5-naphthalenedisulfonate [1] |
| AHFS/ Drugs.com | International Drug Names |
|
Pregnancy category |
|
|
Routes of administration | Oral [2] |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard ( EPA) | |
| ECHA InfoCard | 100.007.606 |
| Chemical and physical data | |
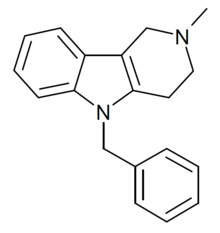
| Formula | C19H20N2 [4] |
| Molar mass | 276.383 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
| | |
Mebhydrolin ( INN) or mebhydroline is an antihistamine. It is not available in the United States, but it is in various other countries under the brand names Bexidal ( BD) and Diazolin ( RU). It is used for symptomatic relief of allergic symptoms caused by histamine release, including nasal allergies and allergic dermatosis.
Mebhydrolin has been shown to magnify the performance-deficit effects of alcohol. [5]
References
- ^ "Mebhydroline". National Library of Medicine - Medical Subject Headings. US National Institutes of Health, National Library of Medicine. Retrieved 2007-03-02.
- ^ a b "FABAHISTIN 50 mg (Tablets)". South African Electronic Package Inserts. September 1970. Archived from the original on October 17, 2006. Retrieved 2007-03-02.
- ^ "Diazoline". National Library of Medicine - Medical Subject Headings. US National Institutes of Health, National Library of Medicine. Retrieved 2007-03-02.
- ^ "Mebhydrolin chemical information". PubChem. Retrieved 2007-03-02.
- ^ Franks HM, Lawrie M, Schabinsky VV, Starmer GA, Teo RK (October 1981). "Interaction between ethanol and antihistamines: 3. mebhydrolin". Med. J. Aust. 2 (9): 477–9. doi: 10.5694/j.1326-5377.1981.tb112944.x. PMID 6119605. S2CID 11034501.
 | |
| Clinical data | |
|---|---|
| Other names | 9-Benzyl-2-methyl-2,3,4,9-tetrahydro-1H-gamma-carboline, Incidal, Omeril, Diazolin, Fabahistin, mebhydrolin napadisylate, mebhydroline 1,5-naphthalenedisulfonate [1] |
| AHFS/ Drugs.com | International Drug Names |
|
Pregnancy category |
|
|
Routes of administration | Oral [2] |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard ( EPA) | |
| ECHA InfoCard | 100.007.606 |
| Chemical and physical data | |
| Formula | C19H20N2 [4] |
| Molar mass | 276.383 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
| | |
Mebhydrolin ( INN) or mebhydroline is an antihistamine. It is not available in the United States, but it is in various other countries under the brand names Bexidal ( BD) and Diazolin ( RU). It is used for symptomatic relief of allergic symptoms caused by histamine release, including nasal allergies and allergic dermatosis.
Mebhydrolin has been shown to magnify the performance-deficit effects of alcohol. [5]
References
- ^ "Mebhydroline". National Library of Medicine - Medical Subject Headings. US National Institutes of Health, National Library of Medicine. Retrieved 2007-03-02.
- ^ a b "FABAHISTIN 50 mg (Tablets)". South African Electronic Package Inserts. September 1970. Archived from the original on October 17, 2006. Retrieved 2007-03-02.
- ^ "Diazoline". National Library of Medicine - Medical Subject Headings. US National Institutes of Health, National Library of Medicine. Retrieved 2007-03-02.
- ^ "Mebhydrolin chemical information". PubChem. Retrieved 2007-03-02.
- ^ Franks HM, Lawrie M, Schabinsky VV, Starmer GA, Teo RK (October 1981). "Interaction between ethanol and antihistamines: 3. mebhydrolin". Med. J. Aust. 2 (9): 477–9. doi: 10.5694/j.1326-5377.1981.tb112944.x. PMID 6119605. S2CID 11034501.