 | |
| Clinical data | |
|---|---|
| Trade names | Gastrozepin |
| AHFS/ Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard ( EPA) | |
| ECHA InfoCard | 100.044.739 |
| Chemical and physical data | |
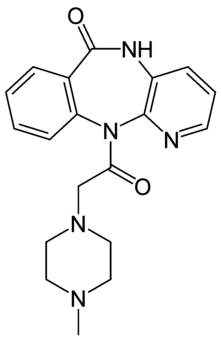
| Formula | C19H21N5O2 |
| Molar mass | 351.410 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
| (verify) | |
Pirenzepine (Gastrozepin), an M1 selective antagonist, is used in the treatment of peptic ulcers, as it reduces gastric acid secretion and reduces muscle spasm. It is in a class of drugs known as muscarinic receptor antagonists; acetylcholine is the neurotransmitter of the parasympathetic nervous system which initiates the rest-and-digest state (as opposed to fight-or-flight), resulting in an increase in gastric motility and digestion; whereas pirenzepine would inhibit these actions and cause decreased gastric motility leading to delayed gastric emptying and constipation. [1] It has no effects on the brain and spinal cord as it cannot diffuse through the blood–brain barrier.
Pirenzepine has been investigated for use in myopia control. [2] [3]
It promotes the homodimerization or oligomerisation of M1 receptors. [4]
- ^ Stolerman IP (2 August 2010). Encyclopedia of Psychopharmacology. Springer. p. 811. ISBN 978-3-540-68698-9. Retrieved 26 June 2013.
- ^ Czepita D (2005). "[Fundamentals of modern treatment of myopia]". Annales Academiae Medicae Stetinensis. 51 (2): 5–9. PMID 16519089.
- ^ Walline JJ, Lindsley KB, Vedula SS, Cotter SA, Mutti DO, Ng SM, Twelker JD (January 2020). "Interventions to slow progression of myopia in children". The Cochrane Database of Systematic Reviews. 1 (1): CD004916. doi: 10.1002/14651858.CD004916.pub4. PMC 6984636. PMID 31930781.
- ^ Pediani JD, Ward RJ, Godin AG, Marsango S, Milligan G (June 2016). "Dynamic Regulation of Quaternary Organization of the M1 Muscarinic Receptor by Subtype-selective Antagonist Drugs". The Journal of Biological Chemistry. 291 (25): 13132–13146. doi: 10.1074/jbc.M115.712562. PMC 4933229. PMID 27080256.
 | |
| Clinical data | |
|---|---|
| Trade names | Gastrozepin |
| AHFS/ Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard ( EPA) | |
| ECHA InfoCard | 100.044.739 |
| Chemical and physical data | |
| Formula | C19H21N5O2 |
| Molar mass | 351.410 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
| (verify) | |
Pirenzepine (Gastrozepin), an M1 selective antagonist, is used in the treatment of peptic ulcers, as it reduces gastric acid secretion and reduces muscle spasm. It is in a class of drugs known as muscarinic receptor antagonists; acetylcholine is the neurotransmitter of the parasympathetic nervous system which initiates the rest-and-digest state (as opposed to fight-or-flight), resulting in an increase in gastric motility and digestion; whereas pirenzepine would inhibit these actions and cause decreased gastric motility leading to delayed gastric emptying and constipation. [1] It has no effects on the brain and spinal cord as it cannot diffuse through the blood–brain barrier.
Pirenzepine has been investigated for use in myopia control. [2] [3]
It promotes the homodimerization or oligomerisation of M1 receptors. [4]
- ^ Stolerman IP (2 August 2010). Encyclopedia of Psychopharmacology. Springer. p. 811. ISBN 978-3-540-68698-9. Retrieved 26 June 2013.
- ^ Czepita D (2005). "[Fundamentals of modern treatment of myopia]". Annales Academiae Medicae Stetinensis. 51 (2): 5–9. PMID 16519089.
- ^ Walline JJ, Lindsley KB, Vedula SS, Cotter SA, Mutti DO, Ng SM, Twelker JD (January 2020). "Interventions to slow progression of myopia in children". The Cochrane Database of Systematic Reviews. 1 (1): CD004916. doi: 10.1002/14651858.CD004916.pub4. PMC 6984636. PMID 31930781.
- ^ Pediani JD, Ward RJ, Godin AG, Marsango S, Milligan G (June 2016). "Dynamic Regulation of Quaternary Organization of the M1 Muscarinic Receptor by Subtype-selective Antagonist Drugs". The Journal of Biological Chemistry. 291 (25): 13132–13146. doi: 10.1074/jbc.M115.712562. PMC 4933229. PMID 27080256.