 | |
| Clinical data | |
|---|---|
| Other names | R-55667; R55667; Tiserton |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard ( EPA) | |
| ECHA InfoCard | 100.163.772 |
| Chemical and physical data | |
| Formula | C27H25F2N3OS |
| Molar mass | 477.57 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
| | |
Ritanserin, also known by its developmental code name R-55667, is a serotonin antagonist medication described as an anxiolytic, antidepressant, antiparkinsonian agent, and antihypertensive agent. [1] [2] [3] It was chiefly investigated as a drug to treat insomnia, especially to enhance sleep quality by significantly increasing slow wave sleep by virtue of potent and concomitant 5HT2a and 5HT2c antagonism [4] [5]
It was never marketed for medical use due to safety problems but is currently used in scientific research.
Some of the safety liabilities that lead to its discontinuation for the treatment of insomnia has led to its potential repurposing in the field of oncology. Specifically, ritanserin acts as a potent inhibitor of diacylglycerol kinase alpha (DGKα). As such, it may be used to treat certain types of glioblastoma [6] [7] and melanoma. It has also been used as a reference compound to identify putatively more selective and potent DGKα inhibitors to treat these forms of cancer as well as possibly others. [8]
Additionally, ritanserin blocks c-RAF activation and induces apoptotic cell death of non–small cell lung cancer and small cell lung cancer cells. [9]
Pharmacology
Pharmacodynamics
Ritanserin acts as a selective 5-HT2A (Ki = 0.45 nM) and 5-HT2C receptor (Ki = 0.71 nM) antagonist. [10] [11] It has relatively low affinity for the H1, D2, α1-adrenergic, and α2-adrenergic receptors (39-, 77-, 107-, and 166-fold lower relative to 5-HT2A, respectively). [11] The affinity of ritanserin for the 5-HT1A receptor is less than 1 μM. [11] In addition to its affinity for the 5-HT2A and 5-HT2C receptors, ritanserin also binds to and antagonizes the 5-HT1D, 5-HT2B, 5-HT5A, 5-HT6, and 5-HT7 receptors. [12]
History
The atypical antipsychotic risperidone was developed from ritanserin. [13]
Society and culture
Names
Ritanserin is the generic name of the drug and its INN, USAN, and BAN. [2] [1] It is also known by its developmental code name R-55667. [1]
Availability
Ritanserin was never approved or marketed for medical use. [14] [15] [16]
Research
Ritanserin was tested in clinical trials for depression, [3] anxiety, schizophrenia, [10] and migraine. [17] It was also found to improve sleep in human volunteers. [16]
Synthesis
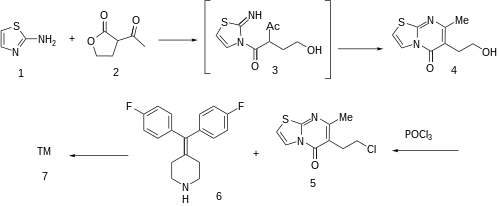
Aminothiazole (2-thiazolamine) (1) is condensed with 2-acetylbutyrolactone [517-23-7] (2) under DS-trap until the water has separated. Condensation of this β-keto lactone can be visualized to involve initial attack on the reactive butyrolactone by the primary nitrogen; cyclodehydration of that hypothetical intermediate 3 gives 6-(2-hydroxyethyl)-7-methyl-[1,3]thiazolo[3,2-a]pyrimidin-5-one, CID:82612453 (4). Halogenation of the terminal alcohol with phosphorus oxychloride then yields 6-(2-chloroethyl)- 7-methyl-5H-thiazolo[3,2-a]pyrimidin-5-one, [86488-00-8] (5). Alkylation with 4-(bis(4-fluorophenyl)methylene)piperidine, [58113-36-3] (6) would complete the synthesis of ritanserin (7).
See also
References
- ^ a b c Elks J, ed. (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. p. 411. ISBN 978-1-4757-2085-3. OCLC 1058412474.
- ^ a b Morton I, Morton IK, Hall JM (31 October 1999). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 249–. ISBN 978-0-7514-0499-9.
- ^ a b Alpert JE, Fava M, Alpert JE, eds. (14 May 2014). Handbook of Chronic Depression: Diagnosis and Therapeutic Management. CRC Press. pp. 117–. ISBN 978-0-8247-5660-4.
- ^ Paiva T, Arriaga F, Wauquier A, Lara E, Largo R, Leitao JN (1988). "Effects of ritanserin on sleep disturbances of dysthymic patients". Psychopharmacology. 96 (3): 395–9. doi: 10.1007/BF00216069. PMID 3146774. S2CID 19232592.
- ^ Idzikowski C, Mills FJ, Glennard R (July 1986). "5-Hydroxytryptamine-2 antagonist increases human slow wave sleep". Brain Research. 378 (1): 164–8. doi: 10.1016/0006-8993(86)90299-4. PMID 3091188. S2CID 43604995.
- ^ Olmez I, Love S, Xiao A, Manigat L, Randolph P, McKenna BD, et al. (January 2018). "Targeting the mesenchymal subtype in glioblastoma and other cancers via inhibition of diacylglycerol kinase alpha". Neuro-Oncology. 20 (2): 192–202. doi: 10.1093/neuonc/nox119. PMC 5777487. PMID 29048560.
- ^ Audia A, Bhat KP (January 2018). "Ritanserin, a novel agent targeting the mesenchymal subtype of glioblastomas". Neuro-Oncology. 20 (2): 151–152. doi: 10.1093/neuonc/nox240. PMC 5786216. PMID 29365204.
- ^ Granade ME, Manigat LC, Lemke MC, Purow BW, Harris TE (March 2022). "Identification of ritanserin analogs that display DGK isoform specificity". Biochemical Pharmacology. 197: 114908. doi: 10.1016/j.bcp.2022.114908. PMC 8858877. PMID 34999054.
- ^ Campbell ST, Franks CE, Borne AL, Shin M, Zhang L, Hsu KL (November 2018). "Chemoproteomic Discovery of a Ritanserin-Targeted Kinase Network Mediating Apoptotic Cell Death of Lung Tumor Cells". Molecular Pharmacology. 94 (5): 1246–1255. doi: 10.1124/mol.118.113001. PMC 6160665. PMID 30158316.
- ^ a b Akhondzadeh S, Malek-Hosseini M, Ghoreishi A, Raznahan M, Rezazadeh SA (December 2008). "Effect of ritanserin, a 5HT2A/2C antagonist, on negative symptoms of schizophrenia: a double-blind randomized placebo-controlled study". Progress in Neuro-Psychopharmacology & Biological Psychiatry. 32 (8): 1879–1883. doi: 10.1016/j.pnpbp.2008.08.020. PMID 18801405. S2CID 12270281.
- ^ a b c Leysen JE, Gommeren W, Van Gompel P, Wynants J, Janssen PF, Laduron PM (June 1985). "Receptor-binding properties in vitro and in vivo of ritanserin: A very potent and long acting serotonin-S2 antagonist". Molecular Pharmacology. 27 (6): 600–611. PMID 2860558.
- ^ Harmful Non-Indigenous Species in the United States. DIANE Publishing. 1 February 1993. pp. 361–. ISBN 978-0-7881-0441-1.
- ^ Lowe III JA (May 1994). "Atypical Antipyschotics based on the D2/5-HT2 ratio hypothesis". Current Medicinal Chemistry. Bentham Science Publishers. pp. 52–.
- ^ "Micromedex Products: Please Login".
- ^ Swiss Pharmaceutical Society (2000). Swiss Pharmaceutical Society (ed.). Index Nominum 2000: International Drug Directory. Taylor & Francis. ISBN 978-3-88763-075-1.
- ^ a b Atkin T, Comai S, Gobbi G (April 2018). "Drugs for Insomnia beyond Benzodiazepines: Pharmacology, Clinical Applications, and Discovery". Pharmacological Reviews. 70 (2): 197–245. doi: 10.1124/pr.117.014381. PMID 29487083. S2CID 3578916.
- ^ Nappi G, Sandrini G, Granella F, Ruiz L, Cerutti G, Facchinetti F, et al. (June 1990). "A new 5-HT2 antagonist (ritanserin) in the treatment of chronic headache with depression. A double-blind study vs amitriptyline". Headache. 30 (7): 439–444. doi: 10.1111/j.1526-4610.1990.hed3007439.x. hdl: 11380/740716. PMID 2119355. S2CID 25781431.
- ^ Prous J, Castaner J (May 1986). "Ritanserin". Drugs of the Future. 11 (5): 391. doi: 10.1358/dof.1986.011.05.50826.
- ^ US 4485107, Kennis LE, Vandenberk J, Mertens JC, issued 1984, assigned to Janssen Pharmaceutica N.V.
- ^ EP 0110435, Kennis LE, Vandenberk J, Mertens JC, issued 1989, assigned to Janssen Pharmaceutica N.V.
 | |
| Clinical data | |
|---|---|
| Other names | R-55667; R55667; Tiserton |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard ( EPA) | |
| ECHA InfoCard | 100.163.772 |
| Chemical and physical data | |
| Formula | C27H25F2N3OS |
| Molar mass | 477.57 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
| | |
Ritanserin, also known by its developmental code name R-55667, is a serotonin antagonist medication described as an anxiolytic, antidepressant, antiparkinsonian agent, and antihypertensive agent. [1] [2] [3] It was chiefly investigated as a drug to treat insomnia, especially to enhance sleep quality by significantly increasing slow wave sleep by virtue of potent and concomitant 5HT2a and 5HT2c antagonism [4] [5]
It was never marketed for medical use due to safety problems but is currently used in scientific research.
Some of the safety liabilities that lead to its discontinuation for the treatment of insomnia has led to its potential repurposing in the field of oncology. Specifically, ritanserin acts as a potent inhibitor of diacylglycerol kinase alpha (DGKα). As such, it may be used to treat certain types of glioblastoma [6] [7] and melanoma. It has also been used as a reference compound to identify putatively more selective and potent DGKα inhibitors to treat these forms of cancer as well as possibly others. [8]
Additionally, ritanserin blocks c-RAF activation and induces apoptotic cell death of non–small cell lung cancer and small cell lung cancer cells. [9]
Pharmacology
Pharmacodynamics
Ritanserin acts as a selective 5-HT2A (Ki = 0.45 nM) and 5-HT2C receptor (Ki = 0.71 nM) antagonist. [10] [11] It has relatively low affinity for the H1, D2, α1-adrenergic, and α2-adrenergic receptors (39-, 77-, 107-, and 166-fold lower relative to 5-HT2A, respectively). [11] The affinity of ritanserin for the 5-HT1A receptor is less than 1 μM. [11] In addition to its affinity for the 5-HT2A and 5-HT2C receptors, ritanserin also binds to and antagonizes the 5-HT1D, 5-HT2B, 5-HT5A, 5-HT6, and 5-HT7 receptors. [12]
History
The atypical antipsychotic risperidone was developed from ritanserin. [13]
Society and culture
Names
Ritanserin is the generic name of the drug and its INN, USAN, and BAN. [2] [1] It is also known by its developmental code name R-55667. [1]
Availability
Ritanserin was never approved or marketed for medical use. [14] [15] [16]
Research
Ritanserin was tested in clinical trials for depression, [3] anxiety, schizophrenia, [10] and migraine. [17] It was also found to improve sleep in human volunteers. [16]
Synthesis

Aminothiazole (2-thiazolamine) (1) is condensed with 2-acetylbutyrolactone [517-23-7] (2) under DS-trap until the water has separated. Condensation of this β-keto lactone can be visualized to involve initial attack on the reactive butyrolactone by the primary nitrogen; cyclodehydration of that hypothetical intermediate 3 gives 6-(2-hydroxyethyl)-7-methyl-[1,3]thiazolo[3,2-a]pyrimidin-5-one, CID:82612453 (4). Halogenation of the terminal alcohol with phosphorus oxychloride then yields 6-(2-chloroethyl)- 7-methyl-5H-thiazolo[3,2-a]pyrimidin-5-one, [86488-00-8] (5). Alkylation with 4-(bis(4-fluorophenyl)methylene)piperidine, [58113-36-3] (6) would complete the synthesis of ritanserin (7).
See also
References
- ^ a b c Elks J, ed. (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. p. 411. ISBN 978-1-4757-2085-3. OCLC 1058412474.
- ^ a b Morton I, Morton IK, Hall JM (31 October 1999). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 249–. ISBN 978-0-7514-0499-9.
- ^ a b Alpert JE, Fava M, Alpert JE, eds. (14 May 2014). Handbook of Chronic Depression: Diagnosis and Therapeutic Management. CRC Press. pp. 117–. ISBN 978-0-8247-5660-4.
- ^ Paiva T, Arriaga F, Wauquier A, Lara E, Largo R, Leitao JN (1988). "Effects of ritanserin on sleep disturbances of dysthymic patients". Psychopharmacology. 96 (3): 395–9. doi: 10.1007/BF00216069. PMID 3146774. S2CID 19232592.
- ^ Idzikowski C, Mills FJ, Glennard R (July 1986). "5-Hydroxytryptamine-2 antagonist increases human slow wave sleep". Brain Research. 378 (1): 164–8. doi: 10.1016/0006-8993(86)90299-4. PMID 3091188. S2CID 43604995.
- ^ Olmez I, Love S, Xiao A, Manigat L, Randolph P, McKenna BD, et al. (January 2018). "Targeting the mesenchymal subtype in glioblastoma and other cancers via inhibition of diacylglycerol kinase alpha". Neuro-Oncology. 20 (2): 192–202. doi: 10.1093/neuonc/nox119. PMC 5777487. PMID 29048560.
- ^ Audia A, Bhat KP (January 2018). "Ritanserin, a novel agent targeting the mesenchymal subtype of glioblastomas". Neuro-Oncology. 20 (2): 151–152. doi: 10.1093/neuonc/nox240. PMC 5786216. PMID 29365204.
- ^ Granade ME, Manigat LC, Lemke MC, Purow BW, Harris TE (March 2022). "Identification of ritanserin analogs that display DGK isoform specificity". Biochemical Pharmacology. 197: 114908. doi: 10.1016/j.bcp.2022.114908. PMC 8858877. PMID 34999054.
- ^ Campbell ST, Franks CE, Borne AL, Shin M, Zhang L, Hsu KL (November 2018). "Chemoproteomic Discovery of a Ritanserin-Targeted Kinase Network Mediating Apoptotic Cell Death of Lung Tumor Cells". Molecular Pharmacology. 94 (5): 1246–1255. doi: 10.1124/mol.118.113001. PMC 6160665. PMID 30158316.
- ^ a b Akhondzadeh S, Malek-Hosseini M, Ghoreishi A, Raznahan M, Rezazadeh SA (December 2008). "Effect of ritanserin, a 5HT2A/2C antagonist, on negative symptoms of schizophrenia: a double-blind randomized placebo-controlled study". Progress in Neuro-Psychopharmacology & Biological Psychiatry. 32 (8): 1879–1883. doi: 10.1016/j.pnpbp.2008.08.020. PMID 18801405. S2CID 12270281.
- ^ a b c Leysen JE, Gommeren W, Van Gompel P, Wynants J, Janssen PF, Laduron PM (June 1985). "Receptor-binding properties in vitro and in vivo of ritanserin: A very potent and long acting serotonin-S2 antagonist". Molecular Pharmacology. 27 (6): 600–611. PMID 2860558.
- ^ Harmful Non-Indigenous Species in the United States. DIANE Publishing. 1 February 1993. pp. 361–. ISBN 978-0-7881-0441-1.
- ^ Lowe III JA (May 1994). "Atypical Antipyschotics based on the D2/5-HT2 ratio hypothesis". Current Medicinal Chemistry. Bentham Science Publishers. pp. 52–.
- ^ "Micromedex Products: Please Login".
- ^ Swiss Pharmaceutical Society (2000). Swiss Pharmaceutical Society (ed.). Index Nominum 2000: International Drug Directory. Taylor & Francis. ISBN 978-3-88763-075-1.
- ^ a b Atkin T, Comai S, Gobbi G (April 2018). "Drugs for Insomnia beyond Benzodiazepines: Pharmacology, Clinical Applications, and Discovery". Pharmacological Reviews. 70 (2): 197–245. doi: 10.1124/pr.117.014381. PMID 29487083. S2CID 3578916.
- ^ Nappi G, Sandrini G, Granella F, Ruiz L, Cerutti G, Facchinetti F, et al. (June 1990). "A new 5-HT2 antagonist (ritanserin) in the treatment of chronic headache with depression. A double-blind study vs amitriptyline". Headache. 30 (7): 439–444. doi: 10.1111/j.1526-4610.1990.hed3007439.x. hdl: 11380/740716. PMID 2119355. S2CID 25781431.
- ^ Prous J, Castaner J (May 1986). "Ritanserin". Drugs of the Future. 11 (5): 391. doi: 10.1358/dof.1986.011.05.50826.
- ^ US 4485107, Kennis LE, Vandenberk J, Mertens JC, issued 1984, assigned to Janssen Pharmaceutica N.V.
- ^ EP 0110435, Kennis LE, Vandenberk J, Mertens JC, issued 1989, assigned to Janssen Pharmaceutica N.V.