 | |
| Clinical data | |
|---|---|
|
Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
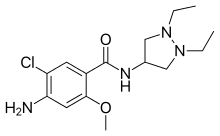
| Formula | C15H23ClN4O2 |
| Molar mass | 326.83 g·mol−1 |
Dazopride (AHR-5531) is an antiemetic and gastroprokinetic agent of the benzamide class which was never marketed. [1] [2] [3] [4] [5] It acts as a 5-HT3 receptor antagonist and 5-HT4 receptor agonist. [3] [4] [6] In addition to its gastrointestinal effects, dazopride facilitates learning and memory in mice. [7]
See also
References
- ^ Grant SC, Kris MG, Gralla RJ, Clark RA, Tyson LB (1993). "Dose-ranging evaluation of the substituted benzamide dazopride when used as an antiemetic in patients receiving anticancer chemotherapy". Cancer Chemotherapy and Pharmacology. 31 (6): 442–444. doi: 10.1007/bf00685032. PMID 8453682. S2CID 23122385.
- ^ Alphin RS, Proakis AG, Leonard CA, Smith WL, Dannenburg WN, Kinnier WJ, et al. (May 1986). "Antagonism of cisplatin-induced emesis by metoclopramide and dazopride through enhancement of gastric motility". Digestive Diseases and Sciences. 31 (5): 524–529. doi: 10.1007/bf01320319. PMID 3698769. S2CID 6571531.
- ^ a b Costall B, Domeney AM, Gunning SJ, Kelly ME, Naylor RJ, Nohria V, et al. (July 1987). "The action of dazopride to enhance gastric emptying and block emesis". Neuropharmacology. 26 (7A): 669–677. doi: 10.1016/0028-3908(87)90227-9. PMID 3114664. S2CID 25143512.
- ^ a b Costall B, Domeney AM, Naylor RJ, Tattersall FD (September 1987). "Emesis induced by cisplatin in the ferret as a model for the detection of anti-emetic drugs". Neuropharmacology. 26 (9): 1321–1326. doi: 10.1016/0028-3908(87)90094-3. PMID 2890117. S2CID 24621209.
- ^ Ganellin CR, Triggle DJ (1996). Dictionary of Pharmacological Agents. Boca Raton: Chapman & Hall/CRC. ISBN 0-412-46630-9.
- ^ Villalón CM, den Boer MO, Heiligers JP, Saxena PR (January 1991). "Further characterization, by use of tryptamine and benzamide derivatives, of the putative 5-HT4 receptor mediating tachycardia in the pig". British Journal of Pharmacology. 102 (1): 107–112. doi: 10.1111/j.1476-5381.1991.tb12140.x. PMC 1917868. PMID 2043916.
- ^ Montgomery SA, Halbreich U (2000). Pharmacotherapy for mood, anxiety, and cognitive disorders. Washington, DC: American Psychiatric Press. ISBN 0-88048-885-9.
Drugs for
functional gastrointestinal disorders (
A03) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drugs for functional bowel disorders |
| ||||||||||||
|
Belladonna and derivatives ( antimuscarinics) |
| ||||||||||||
| Propulsives | |||||||||||||
|
| This drug article relating to the gastrointestinal system is a stub. You can help Wikipedia by expanding it. |
- Articles with short description
- Short description matches Wikidata
- Drugs with non-standard legal status
- Articles without EBI source
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles containing unverified chemical infoboxes
- All stub articles
 | |
| Clinical data | |
|---|---|
|
Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C15H23ClN4O2 |
| Molar mass | 326.83 g·mol−1 |
Dazopride (AHR-5531) is an antiemetic and gastroprokinetic agent of the benzamide class which was never marketed. [1] [2] [3] [4] [5] It acts as a 5-HT3 receptor antagonist and 5-HT4 receptor agonist. [3] [4] [6] In addition to its gastrointestinal effects, dazopride facilitates learning and memory in mice. [7]
See also
References
- ^ Grant SC, Kris MG, Gralla RJ, Clark RA, Tyson LB (1993). "Dose-ranging evaluation of the substituted benzamide dazopride when used as an antiemetic in patients receiving anticancer chemotherapy". Cancer Chemotherapy and Pharmacology. 31 (6): 442–444. doi: 10.1007/bf00685032. PMID 8453682. S2CID 23122385.
- ^ Alphin RS, Proakis AG, Leonard CA, Smith WL, Dannenburg WN, Kinnier WJ, et al. (May 1986). "Antagonism of cisplatin-induced emesis by metoclopramide and dazopride through enhancement of gastric motility". Digestive Diseases and Sciences. 31 (5): 524–529. doi: 10.1007/bf01320319. PMID 3698769. S2CID 6571531.
- ^ a b Costall B, Domeney AM, Gunning SJ, Kelly ME, Naylor RJ, Nohria V, et al. (July 1987). "The action of dazopride to enhance gastric emptying and block emesis". Neuropharmacology. 26 (7A): 669–677. doi: 10.1016/0028-3908(87)90227-9. PMID 3114664. S2CID 25143512.
- ^ a b Costall B, Domeney AM, Naylor RJ, Tattersall FD (September 1987). "Emesis induced by cisplatin in the ferret as a model for the detection of anti-emetic drugs". Neuropharmacology. 26 (9): 1321–1326. doi: 10.1016/0028-3908(87)90094-3. PMID 2890117. S2CID 24621209.
- ^ Ganellin CR, Triggle DJ (1996). Dictionary of Pharmacological Agents. Boca Raton: Chapman & Hall/CRC. ISBN 0-412-46630-9.
- ^ Villalón CM, den Boer MO, Heiligers JP, Saxena PR (January 1991). "Further characterization, by use of tryptamine and benzamide derivatives, of the putative 5-HT4 receptor mediating tachycardia in the pig". British Journal of Pharmacology. 102 (1): 107–112. doi: 10.1111/j.1476-5381.1991.tb12140.x. PMC 1917868. PMID 2043916.
- ^ Montgomery SA, Halbreich U (2000). Pharmacotherapy for mood, anxiety, and cognitive disorders. Washington, DC: American Psychiatric Press. ISBN 0-88048-885-9.
Antiemetics (
A04) | |
|---|---|
|
5-HT3 serotonin ion channel antagonists | |
|
5-HT serotonin G-protein receptor antagonists | |
|
CB1
agonists ( cannabinoids) | |
| D2/D3 antagonists | |
|
H1 antagonists ( antihistamines) | |
|
mACh
antagonists ( anticholinergics) |
|
| NK1 antagonists | |
| Others | |
Drugs for
functional gastrointestinal disorders (
A03) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drugs for functional bowel disorders |
| ||||||||||||
|
Belladonna and derivatives ( antimuscarinics) |
| ||||||||||||
| Propulsives | |||||||||||||
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3– 7 |
| ||||||||||||||||||||||||||||||||||||||
|
| This drug article relating to the gastrointestinal system is a stub. You can help Wikipedia by expanding it. |
- Articles with short description
- Short description matches Wikidata
- Drugs with non-standard legal status
- Articles without EBI source
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles containing unverified chemical infoboxes
- All stub articles