 | |
| Clinical data | |
|---|---|
| Other names | N-[3-[4-(2-Diethylaminoethoxy)phenyl]-1-(dipropylamino)-1-oxopropan-2-yl]benzamide |
| AHFS/ Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard ( EPA) | |
| Chemical and physical data | |
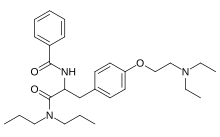
| Formula | C28H41N3O3 |
| Molar mass | 467.654 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
| (verify) | |
Tiropramide is the International nonproprietary name of an antispasmodic drug. [1]
Synthesis
The acylation of racemic tyrosine (1) with benzoyl chloride gives N,O-dibenzoyl-tyrosine (2). Amide formation with dipropylamine (3) using the mixed anhydride method gives the intermediate (4). Hydrolysis of the phenolic ester with sodium hydroxide forms (5), which is alkylated with ClCH2CH2N(CH2CH3)2 to produce the ether tiropramide. [2] [3]
References
- ^ Vidal y Plana RR, Cifarelli A, Setnikar I (January 1981). "Mechanism of smooth muscle relaxation by tiropramide". The Journal of Pharmacy and Pharmacology. 33 (1): 19–24. doi: 10.1111/j.2042-7158.1981.tb13694.x. PMID 6114146. S2CID 22487894.
- ^ Francesco Makovec, Luigi Rovati, Paolo Senin, U.S. patent 4,004,008 (1977 to Rotta Research Laboratorium S.P.A.)
- ^ "Tiropramide". Thieme. Retrieved 2024-07-01.
 | |
| Clinical data | |
|---|---|
| Other names | N-[3-[4-(2-Diethylaminoethoxy)phenyl]-1-(dipropylamino)-1-oxopropan-2-yl]benzamide |
| AHFS/ Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard ( EPA) | |
| Chemical and physical data | |
| Formula | C28H41N3O3 |
| Molar mass | 467.654 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
| (verify) | |
Tiropramide is the International nonproprietary name of an antispasmodic drug. [1]
Synthesis
The acylation of racemic tyrosine (1) with benzoyl chloride gives N,O-dibenzoyl-tyrosine (2). Amide formation with dipropylamine (3) using the mixed anhydride method gives the intermediate (4). Hydrolysis of the phenolic ester with sodium hydroxide forms (5), which is alkylated with ClCH2CH2N(CH2CH3)2 to produce the ether tiropramide. [2] [3]
References
- ^ Vidal y Plana RR, Cifarelli A, Setnikar I (January 1981). "Mechanism of smooth muscle relaxation by tiropramide". The Journal of Pharmacy and Pharmacology. 33 (1): 19–24. doi: 10.1111/j.2042-7158.1981.tb13694.x. PMID 6114146. S2CID 22487894.
- ^ Francesco Makovec, Luigi Rovati, Paolo Senin, U.S. patent 4,004,008 (1977 to Rotta Research Laboratorium S.P.A.)
- ^ "Tiropramide". Thieme. Retrieved 2024-07-01.
