 | |
| Clinical data | |
|---|---|
| AHFS/ Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a601236 |
| ATC code | |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| ChEMBL | |
| CompTox Dashboard ( EPA) | |
| Chemical and physical data | |
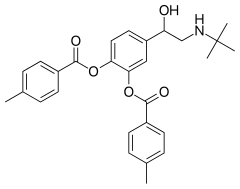
| Formula | C28H31NO5 |
| Molar mass | 461.558 g·mol−1 |
| 3D model ( JSmol) | |
| Chirality | Racemic mixture |
| |
Bitolterol mesylate (Tornalate) is a short-acting β2 adrenergic receptor agonist used for the relief of bronchospasm in conditions such as asthma [1] [2] and COPD. [3] [4] [5] In these disorders there is a narrowing of the airways (bronchi and their ramifications) that carry air to the lungs. Muscle spasm and inflammation within the bronchi worsen this narrowing. Bitolterol relaxes the smooth muscles present continuously around the bronchi and bronchioles facilitating the flow of air through them.

Bitolterol is a prodrug of colterol. [6] [7] It has a rapid onset of action (2–5 minutes) and may last up to 6–8 hours. [8] The drug, alone or in co-administration with theophylline, doesn't show cardiotoxic effect. [9]
The U.S. Food and Drug Administration (FDA) approved bitolterol in December 1984. The drug was withdrawn from the market by Élan Pharmaceuticals in 2001.
References
- ^ Nathan RA, Bodman SF, Storms WW, Mingo TS (June 1986). "Bitolterol mesylate aerosol in adults with steroid-dependent asthma: a comparison with isoproterenol hydrochloride aerosol". Annals of Allergy. 56 (6): 494–499. PMID 3717716.
- ^ Nathan RA, Bernstein IL, Bronsky EA, Bush RK, Chervinsky P, Condemi JJ, et al. (May 1987). "Comparison of the bronchodilator effects of nebulized bitolterol mesylate and isoproterenol hydrochloride in steroid-dependent asthma". The Journal of Allergy and Clinical Immunology. 79 (5): 822–829. doi: 10.1016/0091-6749(87)90216-8. PMID 3571773.
- ^ Friedel HA, Brogden RN (January 1988). "Bitolterol. A preliminary review of its pharmacological properties and therapeutic efficacy in reversible obstructive airways disease". Drugs. 35 (1): 22–41. doi: 10.2165/00003495-198835010-00002. PMID 3278878. S2CID 218471864. Archived from the original on 2012-12-23.
- ^ "Early Career Awards for 1978 (John Robert Anderson, Philip M. Groves, Gary E. Schwartz)". The American Psychologist. 34 (1): 69–79. January 1979. doi: 10.1037/h0078247. PMID 396832.
- ^ Petty TL, Scoggin CH, Rollins DR, Repsher LH (September 1984). "Bitolterol compared to isoproterenol in advanced chronic obstructive pulmonary disease". Chest. 86 (3): 404–408. doi: 10.1378/chest.86.3.404. PMID 6380974.
- ^ Walker SB, Kradjan WA, Bierman CW (6 May 1985). "Bitolterol mesylate: a beta-adrenergic agent. Chemistry, pharmacokinetics, pharmacodynamics, adverse effects and clinical efficacy in asthma". Pharmacotherapy. 5 (3): 127–137. doi: 10.1002/j.1875-9114.1985.tb03410.x. PMID 3895171. S2CID 29431526.
- ^ "ChEBI: Bitolterol". Chemical Entities of Biological Interest. Wellcome Genome Campus, Hinxton, Cambridgeshire, CB10 1SD, UK. Retrieved 27 March 2016.
- ^ Kass I, Mingo TS (August 1980). "Bitolterol mesylate (WIN 32784) aerosol. A new long-acting bronchodilator with reduced chronotropic effects". Chest. 78 (2): 283–287. doi: 10.1378/chest.78.2.283. PMID 6995040.
- ^ Kemp JP, Chervinsky P, Orgel HA, Meltzer EO, Noyes JH, Mingo TS (January 1984). "Concomitant bitolterol mesylate aerosol and theophylline for asthma therapy, with 24 hr electrocardiographic monitoring". The Journal of Allergy and Clinical Immunology. 73 (1 Pt 1): 32–43. doi: 10.1016/0091-6749(84)90481-0. PMID 6693665.
 | |
| Clinical data | |
|---|---|
| AHFS/ Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a601236 |
| ATC code | |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| ChEMBL | |
| CompTox Dashboard ( EPA) | |
| Chemical and physical data | |
| Formula | C28H31NO5 |
| Molar mass | 461.558 g·mol−1 |
| 3D model ( JSmol) | |
| Chirality | Racemic mixture |
| |
Bitolterol mesylate (Tornalate) is a short-acting β2 adrenergic receptor agonist used for the relief of bronchospasm in conditions such as asthma [1] [2] and COPD. [3] [4] [5] In these disorders there is a narrowing of the airways (bronchi and their ramifications) that carry air to the lungs. Muscle spasm and inflammation within the bronchi worsen this narrowing. Bitolterol relaxes the smooth muscles present continuously around the bronchi and bronchioles facilitating the flow of air through them.

Bitolterol is a prodrug of colterol. [6] [7] It has a rapid onset of action (2–5 minutes) and may last up to 6–8 hours. [8] The drug, alone or in co-administration with theophylline, doesn't show cardiotoxic effect. [9]
The U.S. Food and Drug Administration (FDA) approved bitolterol in December 1984. The drug was withdrawn from the market by Élan Pharmaceuticals in 2001.
References
- ^ Nathan RA, Bodman SF, Storms WW, Mingo TS (June 1986). "Bitolterol mesylate aerosol in adults with steroid-dependent asthma: a comparison with isoproterenol hydrochloride aerosol". Annals of Allergy. 56 (6): 494–499. PMID 3717716.
- ^ Nathan RA, Bernstein IL, Bronsky EA, Bush RK, Chervinsky P, Condemi JJ, et al. (May 1987). "Comparison of the bronchodilator effects of nebulized bitolterol mesylate and isoproterenol hydrochloride in steroid-dependent asthma". The Journal of Allergy and Clinical Immunology. 79 (5): 822–829. doi: 10.1016/0091-6749(87)90216-8. PMID 3571773.
- ^ Friedel HA, Brogden RN (January 1988). "Bitolterol. A preliminary review of its pharmacological properties and therapeutic efficacy in reversible obstructive airways disease". Drugs. 35 (1): 22–41. doi: 10.2165/00003495-198835010-00002. PMID 3278878. S2CID 218471864. Archived from the original on 2012-12-23.
- ^ "Early Career Awards for 1978 (John Robert Anderson, Philip M. Groves, Gary E. Schwartz)". The American Psychologist. 34 (1): 69–79. January 1979. doi: 10.1037/h0078247. PMID 396832.
- ^ Petty TL, Scoggin CH, Rollins DR, Repsher LH (September 1984). "Bitolterol compared to isoproterenol in advanced chronic obstructive pulmonary disease". Chest. 86 (3): 404–408. doi: 10.1378/chest.86.3.404. PMID 6380974.
- ^ Walker SB, Kradjan WA, Bierman CW (6 May 1985). "Bitolterol mesylate: a beta-adrenergic agent. Chemistry, pharmacokinetics, pharmacodynamics, adverse effects and clinical efficacy in asthma". Pharmacotherapy. 5 (3): 127–137. doi: 10.1002/j.1875-9114.1985.tb03410.x. PMID 3895171. S2CID 29431526.
- ^ "ChEBI: Bitolterol". Chemical Entities of Biological Interest. Wellcome Genome Campus, Hinxton, Cambridgeshire, CB10 1SD, UK. Retrieved 27 March 2016.
- ^ Kass I, Mingo TS (August 1980). "Bitolterol mesylate (WIN 32784) aerosol. A new long-acting bronchodilator with reduced chronotropic effects". Chest. 78 (2): 283–287. doi: 10.1378/chest.78.2.283. PMID 6995040.
- ^ Kemp JP, Chervinsky P, Orgel HA, Meltzer EO, Noyes JH, Mingo TS (January 1984). "Concomitant bitolterol mesylate aerosol and theophylline for asthma therapy, with 24 hr electrocardiographic monitoring". The Journal of Allergy and Clinical Immunology. 73 (1 Pt 1): 32–43. doi: 10.1016/0091-6749(84)90481-0. PMID 6693665.