 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| Chemical and physical data | |
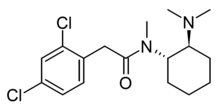
| Formula | C17H24Cl2N2O |
| Molar mass | 343.29 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
U-48800 is an opioid analgesic which has been sold as a designer drug. Unlike U-47700, it is primarily active as a kappa opioid receptor agonist with only moderate affinity at the mu opioid receptor. Nevertheless, it has still appeared on the recreational drug market, often as a component of combinations with other drugs, and has been linked to numerous drug overdose cases. [1] [2] [3] [4] [5]
- ^ Gampfer TM, Richter LH, Schäper J, Wagmann L, Meyer MR (October 2019). "Toxicokinetics and analytical toxicology of the abused opioid U-48800 - in vitro metabolism, metabolic stability, isozyme mapping, and plasma protein binding". Drug Testing and Analysis. 11 (10): 1572–1580. doi: 10.1002/dta.2683. PMID 31424163.
- ^ Baumann MH, Tocco G, Papsun DM, Mohr AL, Fogarty MF, Krotulski AJ (November 2020). "U-47700 and Its Analogs: Non-Fentanyl Synthetic Opioids Impacting the Recreational Drug Market". Brain Sciences. 10 (11): 895. doi: 10.3390/brainsci10110895. PMC 7700279. PMID 33238449.
- ^ Fogarty MF, Mohr AL, Papsun DM, Logan BK (February 2022). "Analysis of the Illicit Opioid U-48800 and Related Compounds by LC-MS-MS and Case Series of Fatalities Involving U-48800". Journal of Analytical Toxicology. 46 (1): 17–24. doi: 10.1093/jat/bkaa180. PMID 33237987.
- ^ Otte L, Wilde M, Auwärter V, Grafinger KE (July 2022). "Investigation of the μ- and κ-opioid receptor activation by eight new synthetic opioids using the [35 S]-GTPγS assay: U-47700, isopropyl U-47700, U-49900, U-47931E, N-methyl U-47931E, U-51754, U-48520, and U-48800". Drug Testing and Analysis. 14 (7): 1187–1199. doi: 10.1002/dta.3238. PMID 35142070.
- ^ Amend N, Thiermann H, Worek F, Wille T (June 2023). "A pharmacologically pre-contracted smooth muscle bowel model for the study of highly-potent opioid receptor agonists and antagonists". Toxicology Letters. 382: 41–46. doi: 10.1016/j.toxlet.2023.05.010. PMID 37245850.
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| Chemical and physical data | |
| Formula | C17H24Cl2N2O |
| Molar mass | 343.29 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
U-48800 is an opioid analgesic which has been sold as a designer drug. Unlike U-47700, it is primarily active as a kappa opioid receptor agonist with only moderate affinity at the mu opioid receptor. Nevertheless, it has still appeared on the recreational drug market, often as a component of combinations with other drugs, and has been linked to numerous drug overdose cases. [1] [2] [3] [4] [5]
- ^ Gampfer TM, Richter LH, Schäper J, Wagmann L, Meyer MR (October 2019). "Toxicokinetics and analytical toxicology of the abused opioid U-48800 - in vitro metabolism, metabolic stability, isozyme mapping, and plasma protein binding". Drug Testing and Analysis. 11 (10): 1572–1580. doi: 10.1002/dta.2683. PMID 31424163.
- ^ Baumann MH, Tocco G, Papsun DM, Mohr AL, Fogarty MF, Krotulski AJ (November 2020). "U-47700 and Its Analogs: Non-Fentanyl Synthetic Opioids Impacting the Recreational Drug Market". Brain Sciences. 10 (11): 895. doi: 10.3390/brainsci10110895. PMC 7700279. PMID 33238449.
- ^ Fogarty MF, Mohr AL, Papsun DM, Logan BK (February 2022). "Analysis of the Illicit Opioid U-48800 and Related Compounds by LC-MS-MS and Case Series of Fatalities Involving U-48800". Journal of Analytical Toxicology. 46 (1): 17–24. doi: 10.1093/jat/bkaa180. PMID 33237987.
- ^ Otte L, Wilde M, Auwärter V, Grafinger KE (July 2022). "Investigation of the μ- and κ-opioid receptor activation by eight new synthetic opioids using the [35 S]-GTPγS assay: U-47700, isopropyl U-47700, U-49900, U-47931E, N-methyl U-47931E, U-51754, U-48520, and U-48800". Drug Testing and Analysis. 14 (7): 1187–1199. doi: 10.1002/dta.3238. PMID 35142070.
- ^ Amend N, Thiermann H, Worek F, Wille T (June 2023). "A pharmacologically pre-contracted smooth muscle bowel model for the study of highly-potent opioid receptor agonists and antagonists". Toxicology Letters. 382: 41–46. doi: 10.1016/j.toxlet.2023.05.010. PMID 37245850.