 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Restas |
| AHFS/ Drugs.com | International Drug Names |
|
Routes of administration | Oral, Intravenous |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 80-90% |
| Metabolism | Hepatic |
| Elimination half-life | 60-90 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard ( EPA) | |
| Chemical and physical data | |
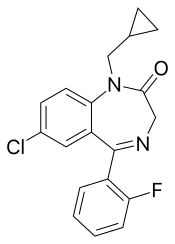
| Formula | C19H16ClFN2O |
| Molar mass | 342.80 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
| | |
Flutoprazepam (Restas) is a drug which is a benzodiazepine. It was patented in Japan by Sumitomo in 1972 [1] and its medical use remains mostly confined to that country. Its muscle relaxant properties are approximately equivalent to those of diazepam - however, it has more powerful sedative, hypnotic, anxiolytic and anticonvulsant effects and is around four times more potent by weight compared to diazepam. [2] It is longer acting than diazepam due to its long-acting active metabolites, [3] which contribute significantly to its effects. [4] Its principal active metabolite is n- desalkylflurazepam, also known as norflurazepam, which is also a principal metabolite of flurazepam (trade name Dalmadorm/Dalmane). [5] [6]
Flutoprazepam is typically used for the treatment of severe insomnia and may also be used for treating stomach ulcers. [7]
Flutoprazepam does not fall under the international Convention on Psychotropic Substances of 1971, and is currently unscheduled in the United States. [8]
- In Singapore, flutoprazepam is a Class C-Schedule II drug under the Misuse of Drugs Act.
- In Thailand, flutoprazepam is a Schedule III psychotropic substance.
- In Hong Kong, flutoprazepam is regulated under Schedule 1 of Hong Kong's Chapter 134 Dangerous Drugs Ordinance. Flutoprazepam can only be used legally by health professionals and for university research purposes. The substance can be given by pharmacists under a prescription. Anyone who supplies the substance without prescription can be fined $10000 (HKD). The penalty for trafficking or manufacturing the substance is a $5,000,000 ( HKD) fine and life imprisonment. Possession of the substance for consumption without license from the Department of Health is illegal with a $1,000,000 (HKD) fine and/or 7 years of jail time. [9]
See also
References
- ^ US patent 3632574, Hisao Yamamoto et al, "PROCESS FOR PRODUCING BENZODIAZEPINE DERIVATIVES", published 1968-29-04, issued 1972-04-01
- ^ Sukamoto T, Aikawa K, Itoh K, Nose T (September 1980). "[Psychopharmacological and general pharmacological studies of 7-chloro-1-cyclopropylmethyl-1, 3-dihydro-5-(2-fluorophenyl)-2H-1, 4-benzodiazepin-2-one (KB-509) (author's transl)]". Nihon Yakurigaku Zasshi. Folia Pharmacologica Japonica. 76 (6): 447–68. doi: 10.1254/fpj.76.447. PMID 7203280.
- ^ Ueki S, Sukamoto T, Watanabe S, Yamamoto T, Kataoka Y, Shibata S, et al. (July 1982). "[Behavioral effects of flutoprazepam (KB-509) and its metabolites]". Nihon Yakurigaku Zasshi. Folia Pharmacologica Japonica. 80 (1): 15–30. doi: 10.1254/fpj.80.15. PMID 6890927.
- ^ Barzaghi N, Leone L, Monteleone M, Tomasini G, Perucca E (1989). "Pharmacokinetics of flutoprazepam, a novel benzodiazepine drug, in normal subjects". European Journal of Drug Metabolism and Pharmacokinetics. 14 (4): 293–8. doi: 10.1007/BF03190114. PMID 2633923. S2CID 20710732.
- ^ Miyaguchi H, Kuwayama K, Tsujikawa K, Kanamori T, Iwata YT, Inoue H, Kishi T (February 2006). "A method for screening for various sedative-hypnotics in serum by liquid chromatography/single quadrupole mass spectrometry". Forensic Science International. 157 (1): 57–70. doi: 10.1016/j.forsciint.2005.03.011. PMID 15869852.
- ^ Barzaghi N, Leone L, Monteleone M, Tomasini G, Perucca E (1989). "Pharmacokinetics of flutoprazepam, a novel benzodiazepine drug, in normal subjects". European Journal of Drug Metabolism and Pharmacokinetics. 14 (4): 293–8. doi: 10.1007/BF03190114. PMID 2633923. S2CID 20710732.
- ^ Fukuda T, Itoh K, Nose T (March 1981). "[Antiulcerogenic action of 7-chloro-1-cyclopropylmethyl-1,3-dihydro-5-(2-fluorophenyl)-2H-1,4-benzodiazepin-2-one (KB-509), a new benzodiazepine derivative]". Nihon Yakurigaku Zasshi. Folia Pharmacologica Japonica. 77 (3): 273–80. doi: 10.1254/fpj.77.273. PMID 7052359.
- ^ "Green List—List of psychotropic substances under international control" (PDF) (26th ed.). International Narcotics Control Board. August 2016. Archived from the original (PDF) on 2017-04-21. Retrieved 2017-07-30.
- ^ "Bilingual Laws Information System" (English). The Government of the Hong Kong Special Administrative Region of the People's Republic of China.
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Restas |
| AHFS/ Drugs.com | International Drug Names |
|
Routes of administration | Oral, Intravenous |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 80-90% |
| Metabolism | Hepatic |
| Elimination half-life | 60-90 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard ( EPA) | |
| Chemical and physical data | |
| Formula | C19H16ClFN2O |
| Molar mass | 342.80 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
| | |
Flutoprazepam (Restas) is a drug which is a benzodiazepine. It was patented in Japan by Sumitomo in 1972 [1] and its medical use remains mostly confined to that country. Its muscle relaxant properties are approximately equivalent to those of diazepam - however, it has more powerful sedative, hypnotic, anxiolytic and anticonvulsant effects and is around four times more potent by weight compared to diazepam. [2] It is longer acting than diazepam due to its long-acting active metabolites, [3] which contribute significantly to its effects. [4] Its principal active metabolite is n- desalkylflurazepam, also known as norflurazepam, which is also a principal metabolite of flurazepam (trade name Dalmadorm/Dalmane). [5] [6]
Flutoprazepam is typically used for the treatment of severe insomnia and may also be used for treating stomach ulcers. [7]
Flutoprazepam does not fall under the international Convention on Psychotropic Substances of 1971, and is currently unscheduled in the United States. [8]
- In Singapore, flutoprazepam is a Class C-Schedule II drug under the Misuse of Drugs Act.
- In Thailand, flutoprazepam is a Schedule III psychotropic substance.
- In Hong Kong, flutoprazepam is regulated under Schedule 1 of Hong Kong's Chapter 134 Dangerous Drugs Ordinance. Flutoprazepam can only be used legally by health professionals and for university research purposes. The substance can be given by pharmacists under a prescription. Anyone who supplies the substance without prescription can be fined $10000 (HKD). The penalty for trafficking or manufacturing the substance is a $5,000,000 ( HKD) fine and life imprisonment. Possession of the substance for consumption without license from the Department of Health is illegal with a $1,000,000 (HKD) fine and/or 7 years of jail time. [9]
See also
References
- ^ US patent 3632574, Hisao Yamamoto et al, "PROCESS FOR PRODUCING BENZODIAZEPINE DERIVATIVES", published 1968-29-04, issued 1972-04-01
- ^ Sukamoto T, Aikawa K, Itoh K, Nose T (September 1980). "[Psychopharmacological and general pharmacological studies of 7-chloro-1-cyclopropylmethyl-1, 3-dihydro-5-(2-fluorophenyl)-2H-1, 4-benzodiazepin-2-one (KB-509) (author's transl)]". Nihon Yakurigaku Zasshi. Folia Pharmacologica Japonica. 76 (6): 447–68. doi: 10.1254/fpj.76.447. PMID 7203280.
- ^ Ueki S, Sukamoto T, Watanabe S, Yamamoto T, Kataoka Y, Shibata S, et al. (July 1982). "[Behavioral effects of flutoprazepam (KB-509) and its metabolites]". Nihon Yakurigaku Zasshi. Folia Pharmacologica Japonica. 80 (1): 15–30. doi: 10.1254/fpj.80.15. PMID 6890927.
- ^ Barzaghi N, Leone L, Monteleone M, Tomasini G, Perucca E (1989). "Pharmacokinetics of flutoprazepam, a novel benzodiazepine drug, in normal subjects". European Journal of Drug Metabolism and Pharmacokinetics. 14 (4): 293–8. doi: 10.1007/BF03190114. PMID 2633923. S2CID 20710732.
- ^ Miyaguchi H, Kuwayama K, Tsujikawa K, Kanamori T, Iwata YT, Inoue H, Kishi T (February 2006). "A method for screening for various sedative-hypnotics in serum by liquid chromatography/single quadrupole mass spectrometry". Forensic Science International. 157 (1): 57–70. doi: 10.1016/j.forsciint.2005.03.011. PMID 15869852.
- ^ Barzaghi N, Leone L, Monteleone M, Tomasini G, Perucca E (1989). "Pharmacokinetics of flutoprazepam, a novel benzodiazepine drug, in normal subjects". European Journal of Drug Metabolism and Pharmacokinetics. 14 (4): 293–8. doi: 10.1007/BF03190114. PMID 2633923. S2CID 20710732.
- ^ Fukuda T, Itoh K, Nose T (March 1981). "[Antiulcerogenic action of 7-chloro-1-cyclopropylmethyl-1,3-dihydro-5-(2-fluorophenyl)-2H-1,4-benzodiazepin-2-one (KB-509), a new benzodiazepine derivative]". Nihon Yakurigaku Zasshi. Folia Pharmacologica Japonica. 77 (3): 273–80. doi: 10.1254/fpj.77.273. PMID 7052359.
- ^ "Green List—List of psychotropic substances under international control" (PDF) (26th ed.). International Narcotics Control Board. August 2016. Archived from the original (PDF) on 2017-04-21. Retrieved 2017-07-30.
- ^ "Bilingual Laws Information System" (English). The Government of the Hong Kong Special Administrative Region of the People's Republic of China.