 | |
| Clinical data | |
|---|---|
| AHFS/ Drugs.com | Micromedex Detailed Consumer Information |
|
Routes of administration | Oral |
| ATC code | |
| Pharmacokinetic data | |
| Excretion | Renal (76%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard ( EPA) | |
| ECHA InfoCard | 100.046.975 |
| Chemical and physical data | |
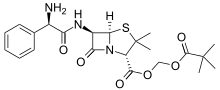
| Formula | C22H29N3O6S |
| Molar mass | 463.55 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
| | |
Pivampicillin is a pivaloyloxymethyl ester of ampicillin. It is a prodrug, which is thought to enhance the oral bioavailability of ampicillin because of its greater lipophilicity compared to that of ampicillin.
Adverse effects
Prodrugs that release pivalic acid when broken down by the body—such as pivampicillin, pivmecillinam, and cefditoren pivoxil—have long been known to deplete levels of carnitine. [1] [2] This effect is not due to the drug itself but to pivalate, which is mostly removed from the body by forming a conjugate with carnitine. Although short-term use of these drugs can cause a marked decrease in blood levels of carnitine, [3] it is unlikely to be of clinical significance; [2] long-term use, however, is not recommended. [2] [4] [5]
Availability
Worldwide, pivampicillin is only available in Denmark, where it is sold as Pondocillin® by PharmaCoDane, or Miraxid® by LEO Pharma. [6]
References
- ^ Holme E, Greter J, Jacobson CE, Lindstedt S, Nordin I, Kristiansson B, Jodal U (August 1989). "Carnitine deficiency induced by pivampicillin and pivmecillinam therapy". Lancet. 2 (8661): 469–473. doi: 10.1016/S0140-6736(89)92086-2. PMID 2570185. S2CID 31555161.
- ^ a b c Brass EP (December 2002). "Pivalate-generating prodrugs and carnitine homeostasis in man". Pharmacological Reviews. 54 (4): 589–598. doi: 10.1124/pr.54.4.589. PMID 12429869. S2CID 14507215.
- ^ Abrahamsson K, Holme E, Jodal U, Lindstedt S, Nordin I (June 1995). "Effect of short-term treatment with pivalic acid containing antibiotics on serum carnitine concentration--a risk irrespective of age". Biochemical and Molecular Medicine. 55 (1): 77–79. doi: 10.1006/bmme.1995.1036. PMID 7551831.
- ^ Holme E, Jodal U, Linstedt S, Nordin I (September 1992). "Effects of pivalic acid-containing prodrugs on carnitine homeostasis and on response to fasting in children". Scandinavian Journal of Clinical and Laboratory Investigation. 52 (5): 361–372. doi: 10.3109/00365519209088371. PMID 1514015.
- ^ Makino Y, Sugiura T, Ito T, Sugiyama N, Koyama N (September 2007). "Carnitine-associated encephalopathy caused by long-term treatment with an antibiotic containing pivalic acid". Pediatrics. 120 (3): e739–e741. doi: 10.1542/peds.2007-0339. PMID 17724113. S2CID 40136171.
- ^ "Pondocillin®". Retrieved 2016-09-18.
 | |
| Clinical data | |
|---|---|
| AHFS/ Drugs.com | Micromedex Detailed Consumer Information |
|
Routes of administration | Oral |
| ATC code | |
| Pharmacokinetic data | |
| Excretion | Renal (76%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard ( EPA) | |
| ECHA InfoCard | 100.046.975 |
| Chemical and physical data | |
| Formula | C22H29N3O6S |
| Molar mass | 463.55 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
| | |
Pivampicillin is a pivaloyloxymethyl ester of ampicillin. It is a prodrug, which is thought to enhance the oral bioavailability of ampicillin because of its greater lipophilicity compared to that of ampicillin.
Adverse effects
Prodrugs that release pivalic acid when broken down by the body—such as pivampicillin, pivmecillinam, and cefditoren pivoxil—have long been known to deplete levels of carnitine. [1] [2] This effect is not due to the drug itself but to pivalate, which is mostly removed from the body by forming a conjugate with carnitine. Although short-term use of these drugs can cause a marked decrease in blood levels of carnitine, [3] it is unlikely to be of clinical significance; [2] long-term use, however, is not recommended. [2] [4] [5]
Availability
Worldwide, pivampicillin is only available in Denmark, where it is sold as Pondocillin® by PharmaCoDane, or Miraxid® by LEO Pharma. [6]
References
- ^ Holme E, Greter J, Jacobson CE, Lindstedt S, Nordin I, Kristiansson B, Jodal U (August 1989). "Carnitine deficiency induced by pivampicillin and pivmecillinam therapy". Lancet. 2 (8661): 469–473. doi: 10.1016/S0140-6736(89)92086-2. PMID 2570185. S2CID 31555161.
- ^ a b c Brass EP (December 2002). "Pivalate-generating prodrugs and carnitine homeostasis in man". Pharmacological Reviews. 54 (4): 589–598. doi: 10.1124/pr.54.4.589. PMID 12429869. S2CID 14507215.
- ^ Abrahamsson K, Holme E, Jodal U, Lindstedt S, Nordin I (June 1995). "Effect of short-term treatment with pivalic acid containing antibiotics on serum carnitine concentration--a risk irrespective of age". Biochemical and Molecular Medicine. 55 (1): 77–79. doi: 10.1006/bmme.1995.1036. PMID 7551831.
- ^ Holme E, Jodal U, Linstedt S, Nordin I (September 1992). "Effects of pivalic acid-containing prodrugs on carnitine homeostasis and on response to fasting in children". Scandinavian Journal of Clinical and Laboratory Investigation. 52 (5): 361–372. doi: 10.3109/00365519209088371. PMID 1514015.
- ^ Makino Y, Sugiura T, Ito T, Sugiyama N, Koyama N (September 2007). "Carnitine-associated encephalopathy caused by long-term treatment with an antibiotic containing pivalic acid". Pediatrics. 120 (3): e739–e741. doi: 10.1542/peds.2007-0339. PMID 17724113. S2CID 40136171.
- ^ "Pondocillin®". Retrieved 2016-09-18.