 | |
| Clinical data | |
|---|---|
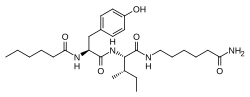
| Other names | N-(1-Oxohexyl)-l-tyrosyl-N-(6-amino-6-oxohexyl)-l-isoleucinamide |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C27H44N4O5 |
| Molar mass | 504.672 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
Dihexa (developmental code name PNB-0408), also known as N-hexanoic-Tyr-Ile-(6) aminohexanoic amide, is an oligopeptide drug derived from angiotensin IV that binds with high affinity to hepatocyte growth factor (HGF) and potentiates its activity at its receptor, c-Met. The compound has been found to potently improve cognitive function in animal models of Alzheimer's disease-like mental impairment. [1] [2] [3] [4] [5] [6] [7] [8] [9] [10] In an assay of neurotrophic activity, Dihexa was found to be seven orders of magnitude more potent than brain-derived neurotrophic factor. [11]
According to a patent, "Short duration safety studies with Dihexa have uncovered no apparent toxicity. Of particular note is a lack of neoplastic induction[ citation needed], since c-Met is recognized as an oncogene. This is unsurprising since oncogenesis requires multiple mutations including both oncogene induction and tumor suppressor attenuation." [12][ citation needed]
History
Dihexa was developed by Joseph Harding and his team at Washington State University. [13] Later developments were done under " M3 Biotechnology", a company founded to commercialise Dihexa. [14]
References
- ^ US 8598118, Harding JW, Wright JW, Benoist CC, Kawas LH, Wayman GA, "Hepatocyte growth factor mimics as therapeutic agents"
- ^ McCoy AT, Benoist CC, Wright JW, Kawas LH, Bule-Ghogare JM, Zhu M, et al. (January 2013). "Evaluation of metabolically stabilized angiotensin IV analogs as procognitive/antidementia agents". The Journal of Pharmacology and Experimental Therapeutics. 344 (1): 141–154. doi: 10.1124/jpet.112.199497. PMC 3533412. PMID 23055539.
- ^ Benoist CC, Kawas LH, Zhu M, Tyson KA, Stillmaker L, Appleyard SM, et al. (November 2014). "The procognitive and synaptogenic effects of angiotensin IV-derived peptides are dependent on activation of the hepatocyte growth factor/c-met system". The Journal of Pharmacology and Experimental Therapeutics. 351 (2): 390–402. doi: 10.1124/jpet.114.218735. PMC 4201273. PMID 25187433.
- ^ Benoist CC, Wright JW, Zhu M, Appleyard SM, Wayman GA, Harding JW (October 2011). "Facilitation of hippocampal synaptogenesis and spatial memory by C-terminal truncated Nle1-angiotensin IV analogs". The Journal of Pharmacology and Experimental Therapeutics. 339 (1): 35–44. doi: 10.1124/jpet.111.182220. PMC 3186286. PMID 21719467.
- ^ Uribe PM, Kawas LH, Harding JW, Coffin AB (January 2015). "Hepatocyte growth factor mimetic protects lateral line hair cells from aminoglycoside exposure". Frontiers in Cellular Neuroscience. 9 (3): 3. doi: 10.3389/fncel.2015.00003. PMC 4309183. PMID 25674052.
- ^ Wright JW, Harding JW (January 2015). "The Brain Hepatocyte Growth Factor/c-Met Receptor System: A New Target for the Treatment of Alzheimer's Disease". Journal of Alzheimer's Disease. 45 (4): 985–1000. doi: 10.3233/JAD-142814. PMID 25649658.
- ^ Siller R, Greenhough S, Naumovska E, Sullivan GJ (May 2015). "Small-molecule-driven hepatocyte differentiation of human pluripotent stem cells". Stem Cell Reports. 4 (5): 939–952. doi: 10.1016/j.stemcr.2015.04.001. PMC 4437467. PMID 25937370.
- ^ "32. The Innovators: Designing Medicine's Holy Grail". KOMO News. 27 August 2015. Retrieved 11 October 2015.
- ^ "Brain Connections in Alzheimer's Rebuilt with New Peptide". GEN News Highlights. 11 October 2015. Retrieved 11 October 2015.
- ^ "Brain-Enhancing 'Smart Drugs' Are Going Commercial". VICE. 17 July 2014. Retrieved 11 October 2015.
- ^ "Prospective Alzheimer's drug builds new brain cell connections, improves cognitive function of rats". ScienceDaily. 11 October 2012. Retrieved 11 October 2015.
- ^ US patent 0337024, Allison Coffin, Joseph Harding, Leen Kawas, Phillip Uribe, "Novel Lead Compound for Otoprotection: Targeting HGF Signaling with Dihexa", issued 2015-11-26
- ^ "Dihexa" (PDF). Alzheimer's Drug Discovery Foundation. August 13, 2021.
- ^ "Fosgonimeton | ALZFORUM". www.alzforum.org. Retrieved 2023-04-20.
 | |
| Clinical data | |
|---|---|
| Other names | N-(1-Oxohexyl)-l-tyrosyl-N-(6-amino-6-oxohexyl)-l-isoleucinamide |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C27H44N4O5 |
| Molar mass | 504.672 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
Dihexa (developmental code name PNB-0408), also known as N-hexanoic-Tyr-Ile-(6) aminohexanoic amide, is an oligopeptide drug derived from angiotensin IV that binds with high affinity to hepatocyte growth factor (HGF) and potentiates its activity at its receptor, c-Met. The compound has been found to potently improve cognitive function in animal models of Alzheimer's disease-like mental impairment. [1] [2] [3] [4] [5] [6] [7] [8] [9] [10] In an assay of neurotrophic activity, Dihexa was found to be seven orders of magnitude more potent than brain-derived neurotrophic factor. [11]
According to a patent, "Short duration safety studies with Dihexa have uncovered no apparent toxicity. Of particular note is a lack of neoplastic induction[ citation needed], since c-Met is recognized as an oncogene. This is unsurprising since oncogenesis requires multiple mutations including both oncogene induction and tumor suppressor attenuation." [12][ citation needed]
History
Dihexa was developed by Joseph Harding and his team at Washington State University. [13] Later developments were done under " M3 Biotechnology", a company founded to commercialise Dihexa. [14]
References
- ^ US 8598118, Harding JW, Wright JW, Benoist CC, Kawas LH, Wayman GA, "Hepatocyte growth factor mimics as therapeutic agents"
- ^ McCoy AT, Benoist CC, Wright JW, Kawas LH, Bule-Ghogare JM, Zhu M, et al. (January 2013). "Evaluation of metabolically stabilized angiotensin IV analogs as procognitive/antidementia agents". The Journal of Pharmacology and Experimental Therapeutics. 344 (1): 141–154. doi: 10.1124/jpet.112.199497. PMC 3533412. PMID 23055539.
- ^ Benoist CC, Kawas LH, Zhu M, Tyson KA, Stillmaker L, Appleyard SM, et al. (November 2014). "The procognitive and synaptogenic effects of angiotensin IV-derived peptides are dependent on activation of the hepatocyte growth factor/c-met system". The Journal of Pharmacology and Experimental Therapeutics. 351 (2): 390–402. doi: 10.1124/jpet.114.218735. PMC 4201273. PMID 25187433.
- ^ Benoist CC, Wright JW, Zhu M, Appleyard SM, Wayman GA, Harding JW (October 2011). "Facilitation of hippocampal synaptogenesis and spatial memory by C-terminal truncated Nle1-angiotensin IV analogs". The Journal of Pharmacology and Experimental Therapeutics. 339 (1): 35–44. doi: 10.1124/jpet.111.182220. PMC 3186286. PMID 21719467.
- ^ Uribe PM, Kawas LH, Harding JW, Coffin AB (January 2015). "Hepatocyte growth factor mimetic protects lateral line hair cells from aminoglycoside exposure". Frontiers in Cellular Neuroscience. 9 (3): 3. doi: 10.3389/fncel.2015.00003. PMC 4309183. PMID 25674052.
- ^ Wright JW, Harding JW (January 2015). "The Brain Hepatocyte Growth Factor/c-Met Receptor System: A New Target for the Treatment of Alzheimer's Disease". Journal of Alzheimer's Disease. 45 (4): 985–1000. doi: 10.3233/JAD-142814. PMID 25649658.
- ^ Siller R, Greenhough S, Naumovska E, Sullivan GJ (May 2015). "Small-molecule-driven hepatocyte differentiation of human pluripotent stem cells". Stem Cell Reports. 4 (5): 939–952. doi: 10.1016/j.stemcr.2015.04.001. PMC 4437467. PMID 25937370.
- ^ "32. The Innovators: Designing Medicine's Holy Grail". KOMO News. 27 August 2015. Retrieved 11 October 2015.
- ^ "Brain Connections in Alzheimer's Rebuilt with New Peptide". GEN News Highlights. 11 October 2015. Retrieved 11 October 2015.
- ^ "Brain-Enhancing 'Smart Drugs' Are Going Commercial". VICE. 17 July 2014. Retrieved 11 October 2015.
- ^ "Prospective Alzheimer's drug builds new brain cell connections, improves cognitive function of rats". ScienceDaily. 11 October 2012. Retrieved 11 October 2015.
- ^ US patent 0337024, Allison Coffin, Joseph Harding, Leen Kawas, Phillip Uribe, "Novel Lead Compound for Otoprotection: Targeting HGF Signaling with Dihexa", issued 2015-11-26
- ^ "Dihexa" (PDF). Alzheimer's Drug Discovery Foundation. August 13, 2021.
- ^ "Fosgonimeton | ALZFORUM". www.alzforum.org. Retrieved 2023-04-20.