| |
| Names | |
|---|---|
|
Preferred IUPAC name
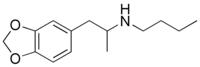
N-[1-(2H-1,3-Benzodioxol-5-yl)propan-2-yl]butan-1-amine | |
| Identifiers | |
3D model (
JSmol)
|
|
| ChemSpider | |
PubChem
CID
|
|
| UNII | |
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C14H21NO2 | |
| Molar mass | 235.327 g·mol−1 |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Methylenedioxybutylamphetamine (MDBU or 3,4-methylenedioxy-N-butylamphetamine) is a lesser-known psychedelic drug. It is also the N-butyl derivative of 3,4-methylenedioxyamphetamine (MDA). MDBU was first synthesized by Alexander Shulgin. In his book PiHKAL, the minimum dosage is listed as 40 mg, and the duration unknown. [1] MDBU produces few to no effects. Very little data exists about the pharmacological properties, metabolism, and toxicity of MDBU.
See also
References

| |
| Names | |
|---|---|
|
Preferred IUPAC name
N-[1-(2H-1,3-Benzodioxol-5-yl)propan-2-yl]butan-1-amine | |
| Identifiers | |
3D model (
JSmol)
|
|
| ChemSpider | |
PubChem
CID
|
|
| UNII | |
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C14H21NO2 | |
| Molar mass | 235.327 g·mol−1 |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Methylenedioxybutylamphetamine (MDBU or 3,4-methylenedioxy-N-butylamphetamine) is a lesser-known psychedelic drug. It is also the N-butyl derivative of 3,4-methylenedioxyamphetamine (MDA). MDBU was first synthesized by Alexander Shulgin. In his book PiHKAL, the minimum dosage is listed as 40 mg, and the duration unknown. [1] MDBU produces few to no effects. Very little data exists about the pharmacological properties, metabolism, and toxicity of MDBU.
See also
References