The hok/sok system is a postsegregational killing mechanism employed by the R1 plasmid in Escherichia coli. It was the first type I toxin-antitoxin pair to be identified through characterisation of a plasmid-stabilising locus. [1] It is a type I system because the toxin is neutralised by a complementary RNA, rather than a partnered protein (type II toxin-antitoxin). [2]
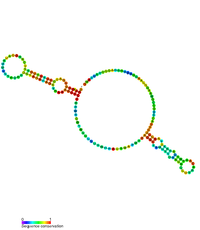
The hok/sok system involves three genes: [3]
- hok, host killing - a long lived ( half-life 20 minutes) toxin
- sok, suppression of killing - a short lived (half-life 30 seconds) RNA antitoxin
- mok, modulation of killing - required for hok translation [4]
| HOK | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | HOK_GEF | ||||||||
| Pfam | PF01848 | ||||||||
| InterPro | IPR000021 | ||||||||
| PROSITE | PDOC00481 | ||||||||
| |||||||||
When E. coli undergoes cell division, the two daughter cells inherit the long-lived hok toxin from the parent cell. Due to the short half-life of the sok antitoxin, daughter cells inherit only small amounts and it quickly degrades. [3]
If a daughter cell has inherited the R1 plasmid, it has inherited the sok gene and a strong promoter which brings about high levels of transcription. So much so that in an R1-positive cell, Sok transcript exists in considerable molar excess over Hok mRNA. [5] Sok RNA then indirectly inhibits the translation of hok by inhibiting mok translation. There is a complementary region where sok transcript binds hok mRNA directly (pictured), but it does not occlude the Shine-Dalgarno sequence. Instead, sok RNA regulates the translation of the mok open reading frame, which nearly entirely overlaps that of hok. It is this translation-coupling which effectively allows sok RNA to repress the translation of hok mRNA. [6]
The sok transcript forms a duplex with the leader region of hok mRNA and this is recognized by RNase III and degraded. The cleavage products are very unstable and soon decay. [7]
Daughter cells without a copy of the R1 plasmid die because they do not have the means to produce more sok antitoxin transcript to inhibit translation of the inherited hok mRNA. The killing system is said to be postsegregational (PSK), [8] since cell death occurs after segregation of the plasmid. [9] [10]
The hok gene codes for a 52 amino acid toxic protein which causes cell death by depolarization of the cell membrane. [11] [12] It works in a similar way to holin proteins which are produced by bacteriophages before cell lysis. [2] [13]
hok/sok homologues denoted flmA/B (FlmA is the protein toxin and FlmB RNA the antisense regulator) [14] are carried on the F plasmid which operate in the same way to maintain the stability of the plasmid. [15] The F plasmid contains another homologous toxin-antitoxin system called srnB. [11]
The first type I toxin-antitoxin system to be found in gram-positive bacteria is the RNAI-RNAII system of the pAD1 plasmid in Enterococcus faecalis. Here, RNAI encodes a toxic protein Fst while RNAII is the regulatory sRNA. [16]
In E. coli strain K-12 there are four long direct repeats (ldr) which encode short open reading frames of 35 codons organised in a homologous manner to the hok/sok system. One of the repeats encodes LdrD, a toxic protein which causes cell death. An unstable antisense RNA regulator (Rd1D) blocks the translation of the LdrD transcript. [17] A mok homologue which overlaps each ldr loci has also been found. [3]
IstR RNA works in a similar system in conjunction with the toxic TisB protein. [18]
- ^ Gerdes K, Larsen JE, Molin S (January 1985). "Stable inheritance of plasmid R1 requires two different loci". J. Bacteriol. 161 (1): 292–8. doi: 10.1128/JB.161.1.292-298.1985. PMC 214870. PMID 2981804.
- ^ a b Hayes F (September 2003). "Toxins-antitoxins: plasmid maintenance, programmed cell death, and cell cycle arrest". Science. 301 (5639): 1496–9. Bibcode: 2003Sci...301.1496H. doi: 10.1126/science.1088157. PMID 12970556. S2CID 10028255.
- ^ a b c Gerdes K, Wagner EG (April 2007). "RNA antitoxins". Curr. Opin. Microbiol. 10 (2): 117–24. doi: 10.1016/j.mib.2007.03.003. PMID 17376733.
- ^ Faridani OR, Nikravesh A, Pandey DP, Gerdes K, Good L (2006). "Competitive inhibition of natural antisense Sok-RNA interactions activates Hok-mediated cell killing in Escherichia coli". Nucleic Acids Res. 34 (20): 5915–22. doi: 10.1093/nar/gkl750. PMC 1635323. PMID 17065468.
- ^ Gerdes K, Thisted T, Martinussen J (November 1990). "Mechanism of post-segregational killing by the hok/sok system of plasmid R1: sok antisense RNA regulates formation of a hok mRNA species correlated with killing of plasmid-free cells". Mol. Microbiol. 4 (11): 1807–18. doi: 10.1111/j.1365-2958.1990.tb02029.x. PMID 1707122. S2CID 45453320.
- ^ Thisted T, Gerdes K (January 1992). "Mechanism of post-segregational killing by the hok/sok system of plasmid R1. Sok antisense RNA regulates hok gene expression indirectly through the overlapping mok gene". J. Mol. Biol. 223 (1): 41–54. doi: 10.1016/0022-2836(92)90714-U. PMID 1370544.
- ^ Gerdes K, Nielsen A, Thorsted P, Wagner EG (August 1992). "Mechanism of killer gene activation. Antisense RNA-dependent RNase III cleavage ensures rapid turn-over of the stable hok, srnB and pndA effector messenger RNAs". J. Mol. Biol. 226 (3): 637–49. doi: 10.1016/0022-2836(92)90621-P. PMID 1380562.
- ^ Gerdes K, Rasmussen PB, Molin S (May 1986). "Unique type of plasmid maintenance function: postsegregational killing of plasmid-free cells". Proc. Natl. Acad. Sci. U.S.A. 83 (10): 3116–20. Bibcode: 1986PNAS...83.3116G. doi: 10.1073/pnas.83.10.3116. PMC 323463. PMID 3517851.
- ^ Thisted T, Sørensen NS, Gerdes K (1995). "Mechanism of post-segregational killing: secondary structure analysis of the entire Hok mRNA from plasmid R1 suggests a fold-back structure that prevents translation and antisense RNA binding". J. Mol. Biol. 247 (5): 859–73. doi: 10.1006/jmbi.1995.0186. PMID 7536849.
- ^ Gerdes K, Bech FW, Jørgensen ST, et al. (August 1986). "Mechanism of postsegregational killing by the hok gene product of the parB system of plasmid R1 and its homology with the relF gene product of the E. coli relB operon". EMBO J. 5 (8): 2023–9. doi: 10.1002/j.1460-2075.1986.tb04459.x. PMC 1167073. PMID 3019679.
- ^ a b Gerdes K, Poulsen LK, Thisted T, Nielsen AK, Martinussen J, Andreasen PH (November 1990). "The hok killer gene family in gram-negative bacteria". New Biol. 2 (11): 946–56. PMID 2101633.
- ^ Pecota DC, Osapay G, Selsted ME, Wood TK (2003). "Antimicrobial properties of the Escherichia coli R1 plasmid host killing peptide". J. Biotechnol. 100 (1): 1–12. doi: 10.1016/S0168-1656(02)00240-7. PMID 12413781.
- ^ Wang IN, Smith DL, Young R (2000). "Holins: the protein clocks of bacteriophage infections". Annu. Rev. Microbiol. 54: 799–825. doi: 10.1146/annurev.micro.54.1.799. PMID 11018145.
- ^ Loh SM, Cram DS, Skurray RA (June 1988). "Nucleotide sequence and transcriptional analysis of a third function (Flm) involved in F-plasmid maintenance". Gene. 66 (2): 259–68. doi: 10.1016/0378-1119(88)90362-9. PMID 3049248.
- ^ Pedersen K, Gerdes K (June 1999). "Multiple hok genes on the chromosome of Escherichia coli". Mol. Microbiol. 32 (5): 1090–102. doi: 10.1046/j.1365-2958.1999.01431.x. PMID 10361310.
- ^ Greenfield TJ, Ehli E, Kirshenmann T, Franch T, Gerdes K, Weaver KE (August 2000). "The antisense RNA of the par locus of pAD1 regulates the expression of a 33-amino-acid toxic peptide by an unusual mechanism". Mol. Microbiol. 37 (3): 652–60. doi: 10.1046/j.1365-2958.2000.02035.x. PMID 10931358.[ dead link]
- ^ Kawano M, Oshima T, Kasai H, Mori H (July 2002). "Molecular characterization of long direct repeat (LDR) sequences expressing a stable mRNA encoding for a 35-amino-acid cell-killing peptide and a cis-encoded small antisense RNA in Escherichia coli". Mol. Microbiol. 45 (2): 333–49. doi: 10.1046/j.1365-2958.2002.03042.x. PMID 12123448.[ dead link]
- ^ Darfeuille F, Unoson C, Vogel J, Wagner EG (May 2007). "An antisense RNA inhibits translation by competing with standby ribosomes". Mol. Cell. 26 (3): 381–92. doi: 10.1016/j.molcel.2007.04.003. PMID 17499044.
- Gerdes K, Gultyaev AP, Franch T, Pedersen K, Mikkelsen ND (1997). "Antisense RNA-regulated programmed cell death". Annu. Rev. Genet. 31: 1–31. doi: 10.1146/annurev.genet.31.1.1. PMID 9442888.
- Poulsen LK, Larsen NW, Molin S, Andersson P (November 1989). "A family of genes encoding a cell-killing function may be conserved in all gram-negative bacteria". Mol. Microbiol. 3 (11): 1463–72. doi: 10.1111/j.1365-2958.1989.tb00131.x. PMID 2693900. S2CID 41192501.
- Nagel JH, Gultyaev AP, Gerdes K, Pleij CW (November 1999). "Metastable structures and refolding kinetics in hok mRNA of plasmid R1". RNA. 5 (11): 1408–18. doi: 10.1017/S1355838299990805. PMC 1369862. PMID 10580469. Retrieved 2010-08-09.
The hok/sok system is a postsegregational killing mechanism employed by the R1 plasmid in Escherichia coli. It was the first type I toxin-antitoxin pair to be identified through characterisation of a plasmid-stabilising locus. [1] It is a type I system because the toxin is neutralised by a complementary RNA, rather than a partnered protein (type II toxin-antitoxin). [2]

The hok/sok system involves three genes: [3]
- hok, host killing - a long lived ( half-life 20 minutes) toxin
- sok, suppression of killing - a short lived (half-life 30 seconds) RNA antitoxin
- mok, modulation of killing - required for hok translation [4]
| HOK | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | HOK_GEF | ||||||||
| Pfam | PF01848 | ||||||||
| InterPro | IPR000021 | ||||||||
| PROSITE | PDOC00481 | ||||||||
| |||||||||
When E. coli undergoes cell division, the two daughter cells inherit the long-lived hok toxin from the parent cell. Due to the short half-life of the sok antitoxin, daughter cells inherit only small amounts and it quickly degrades. [3]
If a daughter cell has inherited the R1 plasmid, it has inherited the sok gene and a strong promoter which brings about high levels of transcription. So much so that in an R1-positive cell, Sok transcript exists in considerable molar excess over Hok mRNA. [5] Sok RNA then indirectly inhibits the translation of hok by inhibiting mok translation. There is a complementary region where sok transcript binds hok mRNA directly (pictured), but it does not occlude the Shine-Dalgarno sequence. Instead, sok RNA regulates the translation of the mok open reading frame, which nearly entirely overlaps that of hok. It is this translation-coupling which effectively allows sok RNA to repress the translation of hok mRNA. [6]
The sok transcript forms a duplex with the leader region of hok mRNA and this is recognized by RNase III and degraded. The cleavage products are very unstable and soon decay. [7]
Daughter cells without a copy of the R1 plasmid die because they do not have the means to produce more sok antitoxin transcript to inhibit translation of the inherited hok mRNA. The killing system is said to be postsegregational (PSK), [8] since cell death occurs after segregation of the plasmid. [9] [10]
The hok gene codes for a 52 amino acid toxic protein which causes cell death by depolarization of the cell membrane. [11] [12] It works in a similar way to holin proteins which are produced by bacteriophages before cell lysis. [2] [13]
hok/sok homologues denoted flmA/B (FlmA is the protein toxin and FlmB RNA the antisense regulator) [14] are carried on the F plasmid which operate in the same way to maintain the stability of the plasmid. [15] The F plasmid contains another homologous toxin-antitoxin system called srnB. [11]
The first type I toxin-antitoxin system to be found in gram-positive bacteria is the RNAI-RNAII system of the pAD1 plasmid in Enterococcus faecalis. Here, RNAI encodes a toxic protein Fst while RNAII is the regulatory sRNA. [16]
In E. coli strain K-12 there are four long direct repeats (ldr) which encode short open reading frames of 35 codons organised in a homologous manner to the hok/sok system. One of the repeats encodes LdrD, a toxic protein which causes cell death. An unstable antisense RNA regulator (Rd1D) blocks the translation of the LdrD transcript. [17] A mok homologue which overlaps each ldr loci has also been found. [3]
IstR RNA works in a similar system in conjunction with the toxic TisB protein. [18]
- ^ Gerdes K, Larsen JE, Molin S (January 1985). "Stable inheritance of plasmid R1 requires two different loci". J. Bacteriol. 161 (1): 292–8. doi: 10.1128/JB.161.1.292-298.1985. PMC 214870. PMID 2981804.
- ^ a b Hayes F (September 2003). "Toxins-antitoxins: plasmid maintenance, programmed cell death, and cell cycle arrest". Science. 301 (5639): 1496–9. Bibcode: 2003Sci...301.1496H. doi: 10.1126/science.1088157. PMID 12970556. S2CID 10028255.
- ^ a b c Gerdes K, Wagner EG (April 2007). "RNA antitoxins". Curr. Opin. Microbiol. 10 (2): 117–24. doi: 10.1016/j.mib.2007.03.003. PMID 17376733.
- ^ Faridani OR, Nikravesh A, Pandey DP, Gerdes K, Good L (2006). "Competitive inhibition of natural antisense Sok-RNA interactions activates Hok-mediated cell killing in Escherichia coli". Nucleic Acids Res. 34 (20): 5915–22. doi: 10.1093/nar/gkl750. PMC 1635323. PMID 17065468.
- ^ Gerdes K, Thisted T, Martinussen J (November 1990). "Mechanism of post-segregational killing by the hok/sok system of plasmid R1: sok antisense RNA regulates formation of a hok mRNA species correlated with killing of plasmid-free cells". Mol. Microbiol. 4 (11): 1807–18. doi: 10.1111/j.1365-2958.1990.tb02029.x. PMID 1707122. S2CID 45453320.
- ^ Thisted T, Gerdes K (January 1992). "Mechanism of post-segregational killing by the hok/sok system of plasmid R1. Sok antisense RNA regulates hok gene expression indirectly through the overlapping mok gene". J. Mol. Biol. 223 (1): 41–54. doi: 10.1016/0022-2836(92)90714-U. PMID 1370544.
- ^ Gerdes K, Nielsen A, Thorsted P, Wagner EG (August 1992). "Mechanism of killer gene activation. Antisense RNA-dependent RNase III cleavage ensures rapid turn-over of the stable hok, srnB and pndA effector messenger RNAs". J. Mol. Biol. 226 (3): 637–49. doi: 10.1016/0022-2836(92)90621-P. PMID 1380562.
- ^ Gerdes K, Rasmussen PB, Molin S (May 1986). "Unique type of plasmid maintenance function: postsegregational killing of plasmid-free cells". Proc. Natl. Acad. Sci. U.S.A. 83 (10): 3116–20. Bibcode: 1986PNAS...83.3116G. doi: 10.1073/pnas.83.10.3116. PMC 323463. PMID 3517851.
- ^ Thisted T, Sørensen NS, Gerdes K (1995). "Mechanism of post-segregational killing: secondary structure analysis of the entire Hok mRNA from plasmid R1 suggests a fold-back structure that prevents translation and antisense RNA binding". J. Mol. Biol. 247 (5): 859–73. doi: 10.1006/jmbi.1995.0186. PMID 7536849.
- ^ Gerdes K, Bech FW, Jørgensen ST, et al. (August 1986). "Mechanism of postsegregational killing by the hok gene product of the parB system of plasmid R1 and its homology with the relF gene product of the E. coli relB operon". EMBO J. 5 (8): 2023–9. doi: 10.1002/j.1460-2075.1986.tb04459.x. PMC 1167073. PMID 3019679.
- ^ a b Gerdes K, Poulsen LK, Thisted T, Nielsen AK, Martinussen J, Andreasen PH (November 1990). "The hok killer gene family in gram-negative bacteria". New Biol. 2 (11): 946–56. PMID 2101633.
- ^ Pecota DC, Osapay G, Selsted ME, Wood TK (2003). "Antimicrobial properties of the Escherichia coli R1 plasmid host killing peptide". J. Biotechnol. 100 (1): 1–12. doi: 10.1016/S0168-1656(02)00240-7. PMID 12413781.
- ^ Wang IN, Smith DL, Young R (2000). "Holins: the protein clocks of bacteriophage infections". Annu. Rev. Microbiol. 54: 799–825. doi: 10.1146/annurev.micro.54.1.799. PMID 11018145.
- ^ Loh SM, Cram DS, Skurray RA (June 1988). "Nucleotide sequence and transcriptional analysis of a third function (Flm) involved in F-plasmid maintenance". Gene. 66 (2): 259–68. doi: 10.1016/0378-1119(88)90362-9. PMID 3049248.
- ^ Pedersen K, Gerdes K (June 1999). "Multiple hok genes on the chromosome of Escherichia coli". Mol. Microbiol. 32 (5): 1090–102. doi: 10.1046/j.1365-2958.1999.01431.x. PMID 10361310.
- ^ Greenfield TJ, Ehli E, Kirshenmann T, Franch T, Gerdes K, Weaver KE (August 2000). "The antisense RNA of the par locus of pAD1 regulates the expression of a 33-amino-acid toxic peptide by an unusual mechanism". Mol. Microbiol. 37 (3): 652–60. doi: 10.1046/j.1365-2958.2000.02035.x. PMID 10931358.[ dead link]
- ^ Kawano M, Oshima T, Kasai H, Mori H (July 2002). "Molecular characterization of long direct repeat (LDR) sequences expressing a stable mRNA encoding for a 35-amino-acid cell-killing peptide and a cis-encoded small antisense RNA in Escherichia coli". Mol. Microbiol. 45 (2): 333–49. doi: 10.1046/j.1365-2958.2002.03042.x. PMID 12123448.[ dead link]
- ^ Darfeuille F, Unoson C, Vogel J, Wagner EG (May 2007). "An antisense RNA inhibits translation by competing with standby ribosomes". Mol. Cell. 26 (3): 381–92. doi: 10.1016/j.molcel.2007.04.003. PMID 17499044.
- Gerdes K, Gultyaev AP, Franch T, Pedersen K, Mikkelsen ND (1997). "Antisense RNA-regulated programmed cell death". Annu. Rev. Genet. 31: 1–31. doi: 10.1146/annurev.genet.31.1.1. PMID 9442888.
- Poulsen LK, Larsen NW, Molin S, Andersson P (November 1989). "A family of genes encoding a cell-killing function may be conserved in all gram-negative bacteria". Mol. Microbiol. 3 (11): 1463–72. doi: 10.1111/j.1365-2958.1989.tb00131.x. PMID 2693900. S2CID 41192501.
- Nagel JH, Gultyaev AP, Gerdes K, Pleij CW (November 1999). "Metastable structures and refolding kinetics in hok mRNA of plasmid R1". RNA. 5 (11): 1408–18. doi: 10.1017/S1355838299990805. PMC 1369862. PMID 10580469. Retrieved 2010-08-09.

