This article needs additional citations for
verification. (April 2015) |

| |
| |
| Names | |
|---|---|
|
IUPAC name
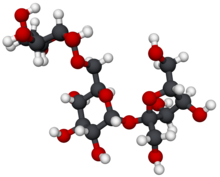
β-D-Fructofuranosyl α-D-galactopyranosyl-(1→6)-α-D-glucopyranoside
| |
|
Systematic IUPAC name
(2R,3R,4S,5S,6R)-2-{[(2S,3S,4S,5R)-3,4-Dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy}-6-({[(2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}methyl)oxane-3,4,5-triol | |
| Other names
rafinosa
D-(+)-Raffinose D-Raffinose D-raffinose pentahydrate Gossypose Melitose Melitriose NSC 170228 NSC 2025 6G-α-D-galactosylsucrose; β-D-fructofuranosyl-O-α-D-glucopyranosyl-(1→6)-α-D-galactopyranoside hydrate(1:5) | |
| Identifiers | |
| |
3D model (
JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.407 |
| EC Number |
|
| KEGG | |
PubChem
CID
|
|
| UNII | |
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C18H32O16 | |
| Molar mass | 594.5 g/mol (pentahydrate) |
| Melting point | 118 °C |
| 203 g/L | |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Raffinose is a trisaccharide composed of galactose, glucose, and fructose. It can be found in beans, cabbage, brussels sprouts, broccoli, asparagus, other vegetables, and whole grains. Raffinose can be hydrolyzed to D-galactose and sucrose by the enzyme α-galactosidase (α-GAL), an enzyme synthesized by bacteria found in the large intestine. α-GAL also hydrolyzes other α-galactosides such as stachyose, verbascose, and galactinol, if present. In plants, raffinose plays a significant role in stress responses, particularly temperature sensitivity, seed vigour, resistance to pathogens, and desiccation.
Chemical properties
The raffinose family of oligosaccharides (RFOs) are α-galactosyl derivatives of sucrose, the most common being the trisaccharide raffinose, the tetrasaccharide stachyose, and the pentasaccharide verbascose. RFOs are almost ubiquitous across the plant kingdom, being found in a large variety of seeds from many different families. They rank second only to sucrose in abundance as soluble carbohydrates. [1]
Raffinose typically crystallises as a pentahydrate white crystalline powder. [2] It is odorless and has a sweet taste approximately 10% that of sucrose. [3]
Biochemical properties
Energy source
It is non-digestible in humans and other monogastric animals ( pigs and poultry) who do not possess the α-GAL enzyme to break down RFOs. These oligosaccharides pass undigested through the stomach and small intestine. In the large intestine, they are fermented by bacteria that do possess the α-GAL enzyme and make short-chain fatty acids (SCFA)(acetic, propionic, butyric acids), as well as the flatulence commonly associated with eating beans and other vegetables. These SCFAs have been recently found to impart a number of health benefits. α-GAL is present in digestive aids such as the product Beano. [4]
Plant Health
Cases of abiotic stress such as temperature, drought, and salinity have shown to increase RFO levels, especially raffinose, in plants. The functional role raffinose plays in abiotic stress tolerance is not well known, but its presence as a positive regulator of these stresses is established. [5]
Galactinol synthase (GolS) is an enzyme key in the synthesis of RFOs. Studies which modify the expression of GolS have been done to understand the role of RFOs in stress response. [6] [7] GolS has been found to induce salicylic acid signalling pathways and expression of defence-related genes, showing RFOs to have a role in pathogen resistance.
RFOs have been seen to assist in seed germination. They are used as a source of energy and carbon for germination, and protect the seed from desiccation during the maturation process. One proposed mechanism of RFOs working against desiccation details the hydroxyl groups replacing water to maintain hydrophilicity ion the cell, which stabilizes the membrane structure and macromolecules needed for cellular function. Another mechanism, called "vitrification", has the cell taking on a highly viscous form, like that of a plastic solid. This maintains cellular stability and hydrogen bonding in the cell, and prevents cellular collapse. [8]
In many plants, RFOs have been seen to act as an alternative to sucrose for sugar storage and transport. [9]
Disease relevance
Research has shown that the differential ability to utilize raffinose by strains of the bacteria Streptococcus pneumoniae, impacts their ability to cause disease and the nature of the disease. [10]
Uses
Procedures concerning cryopreservation have used raffinose to provide hypertonicity for cell desiccation prior to freezing. [11] Either raffinose or sucrose is used as a base substance for sucralose.
Raffinose is also used in:
- skin moisturizers and cosmetics [12]
- prebiotics (it promotes growth of lactobacilli and bifidobacteria) [13] [14]
- food or drinks additive
- chiral stationary phase in HPLC [15]
See also
Further reading
References
- ^ Pontis, Horacio G. (2017-01-01), Pontis, Horacio G. (ed.), "Chapter 8 - Case Study: Raffinose", Methods for Analysis of Carbohydrate Metabolism in Photosynthetic Organisms, Boston: Academic Press, pp. 111–120, doi: 10.1016/b978-0-12-803396-8.00008-9, ISBN 978-0-12-803396-8, retrieved 2024-04-15
- ^ Kawasaki, T.; Takahashi, M.; Kiyanagi, R.; Ohhara, T. (2022-12-01). "Rearrangement of hydrogen bonds in dehydrated raffinose tetrahydrate: a time-of-flight neutron diffraction study". Acta Crystallographica Section C: Structural Chemistry. 78 (12): 743–748. doi: 10.1107/S2053229622010828. ISSN 2053-2296. PMID 36468557. S2CID 253698466.
- ^ "D(+)-Raffinose pentahydrate | 17629-30-0". www.chemicalbook.com. Retrieved 2019-08-19.
- ^ Ganiats, T. G.; Norcross, W. A.; Halverson, A. L.; Burford, P. A.; Palinkas, L. A. (November 1994). "Does Beano prevent gas? A double-blind crossover study of oral alpha-galactosidase to treat dietary oligosaccharide intolerance". The Journal of Family Practice. 39 (5): 441–445. ISSN 0094-3509. PMID 7964541.
- ^ Yan, Shijuan; Liu, Qing; Li, Wenyan; Yan, Jianbing; Fernie, Alisdair R. (2022-07-04). "Raffinose Family Oligosaccharides: Crucial Regulators of Plant Development and Stress Responses". Critical Reviews in Plant Sciences. 41 (4): 286–303. Bibcode: 2022CRvPS..41..286Y. doi: 10.1080/07352689.2022.2111756. ISSN 0735-2689.
- ^ dos Santos, Tiago Benedito; Vieira, Luiz Gonzaga Esteves (2020-12-01). "Involvement of the galactinol synthase gene in abiotic and biotic stress responses: A review on current knowledge". Plant Gene. 24: 100258. doi: 10.1016/j.plgene.2020.100258. ISSN 2352-4073.
- ^ Keunen, Els; Peshev, Darin; Vangronsveld, Jaco; Van Den Ende, Wim; Cuypers, Ann (July 2013). "Plant sugars are crucial players in the oxidative challenge during abiotic stress: extending the traditional concept". Plant, Cell & Environment. 36 (7): 1242–1255. doi: 10.1111/pce.12061. ISSN 0140-7791. PMID 23305614.
- ^ Salvi, Prafull; Varshney, Vishal; Majee, Manoj (October 2022). "Raffinose family oligosaccharides (RFOs): role in seed vigor and longevity". Bioscience Reports. 42 (10). doi: 10.1042/bsr20220198. ISSN 0144-8463. PMC 9547172. PMID 36149314.
- ^ Kanwal, Freeha; Ren, Dingxin; Kanwal, Wajiha; Ding, Mengying; Su, Junqing; Shang, Xiaoya (2023-02-16). "The potential role of nondigestible Raffinose family oligosaccharides as prebiotics". Glycobiology. 33 (4): 274–288. doi: 10.1093/glycob/cwad015. ISSN 1460-2423. PMID 36795047.
- ^ Minhas, Vikrant; Harvey, Richard M.; McAllister, Lauren J.; Seemann, Torsten; Syme, Anna E.; Baines, Sarah L.; Paton, James C.; Trappetti, Claudia (2019-01-15). McDaniel, Larry S. (ed.). "Capacity To Utilize Raffinose Dictates Pneumococcal Disease Phenotype". mBio. 10 (1). doi: 10.1128/mBio.02596-18. ISSN 2150-7511. PMC 6336424. PMID 30647157.
-
^ Storey B., Noiles, E., Thompson, K. (1998).
"Comparison of Glycerol, Other Polyols, Trehalose, and Raffinose to Provide a Defined Cryoprotectant Medium for Mouse Sperm Cryopreservation". Cryobiology. 37 (1): 46–58.
doi:
10.1006/cryo.1998.2097.
PMID
9698429.
{{ cite journal}}: CS1 maint: multiple names: authors list ( link) - ^ Na, Tae-Young; Kim, Gyeong-Hwan; Oh, Hyeon-Jeong; Lee, Min-Ho; Han, Yong-Hyun; Kim, Ki Taek; Kim, Ji-Su; Kim, Dae-Duk; Lee, Mi-Ock (2017-03-07). "The trisaccharide raffinose modulates epidermal differentiation through activation of liver X receptor". Scientific Reports. 7 (1): 43823. Bibcode: 2017NatSR...743823N. doi: 10.1038/srep43823. ISSN 2045-2322. PMC 5339792. PMID 28266648.
- ^ Zartl, Barbara; Silberbauer, Karina; Loeppert, Renate; Viernstein, Helmut; Praznik, Werner; Mueller, Monika (2018-03-21). "Fermentation of non-digestible raffinose family oligosaccharides and galactomannans by probiotics". Food & Function. 9 (3): 1638–1646. doi: 10.1039/C7FO01887H. ISSN 2042-650X. PMID 29465736.
- ^ Anggraeni, A A (2022-02-01). "Mini-Review: The potential of raffinose as a prebiotic". IOP Conference Series: Earth and Environmental Science. 980 (1): 012033. Bibcode: 2022E&ES..980a2033A. doi: 10.1088/1755-1315/980/1/012033. ISSN 1755-1307.
- ^ Qiu, Hongdeng; Liang, Xiaojing; Sun, Min; Jiang, Shengxiang (2011-04-01). "Development of silica-based stationary phases for high-performance liquid chromatography". Analytical and Bioanalytical Chemistry. 399 (10): 3307–3322. doi: 10.1007/s00216-010-4611-x. ISSN 1618-2650. PMID 21221544.
This article needs additional citations for
verification. (April 2015) |

| |

| |
| Names | |
|---|---|
|
IUPAC name
β-D-Fructofuranosyl α-D-galactopyranosyl-(1→6)-α-D-glucopyranoside
| |
|
Systematic IUPAC name
(2R,3R,4S,5S,6R)-2-{[(2S,3S,4S,5R)-3,4-Dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy}-6-({[(2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}methyl)oxane-3,4,5-triol | |
| Other names
rafinosa
D-(+)-Raffinose D-Raffinose D-raffinose pentahydrate Gossypose Melitose Melitriose NSC 170228 NSC 2025 6G-α-D-galactosylsucrose; β-D-fructofuranosyl-O-α-D-glucopyranosyl-(1→6)-α-D-galactopyranoside hydrate(1:5) | |
| Identifiers | |
| |
3D model (
JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.407 |
| EC Number |
|
| KEGG | |
PubChem
CID
|
|
| UNII | |
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C18H32O16 | |
| Molar mass | 594.5 g/mol (pentahydrate) |
| Melting point | 118 °C |
| 203 g/L | |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Raffinose is a trisaccharide composed of galactose, glucose, and fructose. It can be found in beans, cabbage, brussels sprouts, broccoli, asparagus, other vegetables, and whole grains. Raffinose can be hydrolyzed to D-galactose and sucrose by the enzyme α-galactosidase (α-GAL), an enzyme synthesized by bacteria found in the large intestine. α-GAL also hydrolyzes other α-galactosides such as stachyose, verbascose, and galactinol, if present. In plants, raffinose plays a significant role in stress responses, particularly temperature sensitivity, seed vigour, resistance to pathogens, and desiccation.
Chemical properties
The raffinose family of oligosaccharides (RFOs) are α-galactosyl derivatives of sucrose, the most common being the trisaccharide raffinose, the tetrasaccharide stachyose, and the pentasaccharide verbascose. RFOs are almost ubiquitous across the plant kingdom, being found in a large variety of seeds from many different families. They rank second only to sucrose in abundance as soluble carbohydrates. [1]
Raffinose typically crystallises as a pentahydrate white crystalline powder. [2] It is odorless and has a sweet taste approximately 10% that of sucrose. [3]
Biochemical properties
Energy source
It is non-digestible in humans and other monogastric animals ( pigs and poultry) who do not possess the α-GAL enzyme to break down RFOs. These oligosaccharides pass undigested through the stomach and small intestine. In the large intestine, they are fermented by bacteria that do possess the α-GAL enzyme and make short-chain fatty acids (SCFA)(acetic, propionic, butyric acids), as well as the flatulence commonly associated with eating beans and other vegetables. These SCFAs have been recently found to impart a number of health benefits. α-GAL is present in digestive aids such as the product Beano. [4]
Plant Health
Cases of abiotic stress such as temperature, drought, and salinity have shown to increase RFO levels, especially raffinose, in plants. The functional role raffinose plays in abiotic stress tolerance is not well known, but its presence as a positive regulator of these stresses is established. [5]
Galactinol synthase (GolS) is an enzyme key in the synthesis of RFOs. Studies which modify the expression of GolS have been done to understand the role of RFOs in stress response. [6] [7] GolS has been found to induce salicylic acid signalling pathways and expression of defence-related genes, showing RFOs to have a role in pathogen resistance.
RFOs have been seen to assist in seed germination. They are used as a source of energy and carbon for germination, and protect the seed from desiccation during the maturation process. One proposed mechanism of RFOs working against desiccation details the hydroxyl groups replacing water to maintain hydrophilicity ion the cell, which stabilizes the membrane structure and macromolecules needed for cellular function. Another mechanism, called "vitrification", has the cell taking on a highly viscous form, like that of a plastic solid. This maintains cellular stability and hydrogen bonding in the cell, and prevents cellular collapse. [8]
In many plants, RFOs have been seen to act as an alternative to sucrose for sugar storage and transport. [9]
Disease relevance
Research has shown that the differential ability to utilize raffinose by strains of the bacteria Streptococcus pneumoniae, impacts their ability to cause disease and the nature of the disease. [10]
Uses
Procedures concerning cryopreservation have used raffinose to provide hypertonicity for cell desiccation prior to freezing. [11] Either raffinose or sucrose is used as a base substance for sucralose.
Raffinose is also used in:
- skin moisturizers and cosmetics [12]
- prebiotics (it promotes growth of lactobacilli and bifidobacteria) [13] [14]
- food or drinks additive
- chiral stationary phase in HPLC [15]
See also
Further reading
References
- ^ Pontis, Horacio G. (2017-01-01), Pontis, Horacio G. (ed.), "Chapter 8 - Case Study: Raffinose", Methods for Analysis of Carbohydrate Metabolism in Photosynthetic Organisms, Boston: Academic Press, pp. 111–120, doi: 10.1016/b978-0-12-803396-8.00008-9, ISBN 978-0-12-803396-8, retrieved 2024-04-15
- ^ Kawasaki, T.; Takahashi, M.; Kiyanagi, R.; Ohhara, T. (2022-12-01). "Rearrangement of hydrogen bonds in dehydrated raffinose tetrahydrate: a time-of-flight neutron diffraction study". Acta Crystallographica Section C: Structural Chemistry. 78 (12): 743–748. doi: 10.1107/S2053229622010828. ISSN 2053-2296. PMID 36468557. S2CID 253698466.
- ^ "D(+)-Raffinose pentahydrate | 17629-30-0". www.chemicalbook.com. Retrieved 2019-08-19.
- ^ Ganiats, T. G.; Norcross, W. A.; Halverson, A. L.; Burford, P. A.; Palinkas, L. A. (November 1994). "Does Beano prevent gas? A double-blind crossover study of oral alpha-galactosidase to treat dietary oligosaccharide intolerance". The Journal of Family Practice. 39 (5): 441–445. ISSN 0094-3509. PMID 7964541.
- ^ Yan, Shijuan; Liu, Qing; Li, Wenyan; Yan, Jianbing; Fernie, Alisdair R. (2022-07-04). "Raffinose Family Oligosaccharides: Crucial Regulators of Plant Development and Stress Responses". Critical Reviews in Plant Sciences. 41 (4): 286–303. Bibcode: 2022CRvPS..41..286Y. doi: 10.1080/07352689.2022.2111756. ISSN 0735-2689.
- ^ dos Santos, Tiago Benedito; Vieira, Luiz Gonzaga Esteves (2020-12-01). "Involvement of the galactinol synthase gene in abiotic and biotic stress responses: A review on current knowledge". Plant Gene. 24: 100258. doi: 10.1016/j.plgene.2020.100258. ISSN 2352-4073.
- ^ Keunen, Els; Peshev, Darin; Vangronsveld, Jaco; Van Den Ende, Wim; Cuypers, Ann (July 2013). "Plant sugars are crucial players in the oxidative challenge during abiotic stress: extending the traditional concept". Plant, Cell & Environment. 36 (7): 1242–1255. doi: 10.1111/pce.12061. ISSN 0140-7791. PMID 23305614.
- ^ Salvi, Prafull; Varshney, Vishal; Majee, Manoj (October 2022). "Raffinose family oligosaccharides (RFOs): role in seed vigor and longevity". Bioscience Reports. 42 (10). doi: 10.1042/bsr20220198. ISSN 0144-8463. PMC 9547172. PMID 36149314.
- ^ Kanwal, Freeha; Ren, Dingxin; Kanwal, Wajiha; Ding, Mengying; Su, Junqing; Shang, Xiaoya (2023-02-16). "The potential role of nondigestible Raffinose family oligosaccharides as prebiotics". Glycobiology. 33 (4): 274–288. doi: 10.1093/glycob/cwad015. ISSN 1460-2423. PMID 36795047.
- ^ Minhas, Vikrant; Harvey, Richard M.; McAllister, Lauren J.; Seemann, Torsten; Syme, Anna E.; Baines, Sarah L.; Paton, James C.; Trappetti, Claudia (2019-01-15). McDaniel, Larry S. (ed.). "Capacity To Utilize Raffinose Dictates Pneumococcal Disease Phenotype". mBio. 10 (1). doi: 10.1128/mBio.02596-18. ISSN 2150-7511. PMC 6336424. PMID 30647157.
-
^ Storey B., Noiles, E., Thompson, K. (1998).
"Comparison of Glycerol, Other Polyols, Trehalose, and Raffinose to Provide a Defined Cryoprotectant Medium for Mouse Sperm Cryopreservation". Cryobiology. 37 (1): 46–58.
doi:
10.1006/cryo.1998.2097.
PMID
9698429.
{{ cite journal}}: CS1 maint: multiple names: authors list ( link) - ^ Na, Tae-Young; Kim, Gyeong-Hwan; Oh, Hyeon-Jeong; Lee, Min-Ho; Han, Yong-Hyun; Kim, Ki Taek; Kim, Ji-Su; Kim, Dae-Duk; Lee, Mi-Ock (2017-03-07). "The trisaccharide raffinose modulates epidermal differentiation through activation of liver X receptor". Scientific Reports. 7 (1): 43823. Bibcode: 2017NatSR...743823N. doi: 10.1038/srep43823. ISSN 2045-2322. PMC 5339792. PMID 28266648.
- ^ Zartl, Barbara; Silberbauer, Karina; Loeppert, Renate; Viernstein, Helmut; Praznik, Werner; Mueller, Monika (2018-03-21). "Fermentation of non-digestible raffinose family oligosaccharides and galactomannans by probiotics". Food & Function. 9 (3): 1638–1646. doi: 10.1039/C7FO01887H. ISSN 2042-650X. PMID 29465736.
- ^ Anggraeni, A A (2022-02-01). "Mini-Review: The potential of raffinose as a prebiotic". IOP Conference Series: Earth and Environmental Science. 980 (1): 012033. Bibcode: 2022E&ES..980a2033A. doi: 10.1088/1755-1315/980/1/012033. ISSN 1755-1307.
- ^ Qiu, Hongdeng; Liang, Xiaojing; Sun, Min; Jiang, Shengxiang (2011-04-01). "Development of silica-based stationary phases for high-performance liquid chromatography". Analytical and Bioanalytical Chemistry. 399 (10): 3307–3322. doi: 10.1007/s00216-010-4611-x. ISSN 1618-2650. PMID 21221544.