| |
| Identifiers | |
|---|---|
3D model (
JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.033.460 |
| EC Number |
|
PubChem
CID
|
|
| UN number | 3098 [2] |
| |
| Properties | |
| Pr(ClO4)3 | |
| Molar mass | 439.259 [1] |
| Density | 1.563 [2] |
| Melting point | liquid at room temperature [2] |
| Vapor pressure | 0.21 psi (20 °C) [2] |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
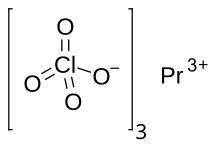
Praseodymium(III) perchlorate is the perchlorate salt of praseodymium, with the chemical formula of Pr(ClO4)3. [3]
Preparation
Praseodymium(III) perchlorate can be prepared from praseodymium(III,IV) oxide. [4] [5] Dissolving praseodymium(III,IV) oxide in a slight excess of hydrochloric acid and adding a small amount of hydrogen peroxide can prepare praseodymium perchlorate. [5]
Chemical properties
Praseodymium perchlorate can form two complexes with crown ether 18-crown-6 in stoichiometric ratios of 1:1 and 1:2., [6] and can form complexes with L- proline, [7] glutamic acid, [4] mandelic acid, [4] penicillamine. [4] It can also form complexes with imidazole and alanine. [5]
References
- ^ a b c "Praseodymium perchlorate". ChemSpider. Retrieved 2017-10-21.
- ^ a b c d e "Praseodymium perchlorate 13498-07-2". www.chemicalbook.com. Retrieved 2017-10-20.
- ^ PubChem. "Praseodymium(3+) perchlorate". pubchem.ncbi.nlm.nih.gov. Retrieved 2022-07-11.
- ^ a b c d 张若桦, 陈敬堂、江冬青 (1995). "镨(III)溶液光谱研究:高氯酸镨─谷氨酸/苦杏仁酸/青霉胺配合物". Acta Scientiarum Naturalium Universitatis Nankaiensis (1): 44-48. ISSN 0465-7942. Retrieved 2017-10-21.
- ^ a b c 但悠梦, 胡卫兵、余华光、董家新、刘义、屈松生 (2006). "稀土配合物[Pr(C3H7NO2)2(C3H4N2)(H2O)](ClO4)3的标准生成焓及热分解动力学研究" [Standard Molar Enthalpy of Formation and Thermal Decomposition Kinetic Study of Rare Earth Complex [Pr(C3H7NO2)2(C3H4N2)(H2O)](ClO4)3]. Acta Chimica Sinica. 64 (1): 70-78. doi: 10.3321/j.issn:0567-7351.2006.01.012. ISSN 0567-7351. S2CID 236111702. Retrieved 2017-10-21.
- ^ Xue Ganglin; Li Qianding; Hu Shaoming; Xu Hong; Ren Dehou; Zhuang Quanzhen (1996). "三水合高氯酸镨与18—冠—6配合物的研究" [Study on complexes of trihydrated praseodymium perchlorate with 18 -crown-6]. Acta Chimica Sinica (in Chinese). 54 (6): 568-574. ISSN 0567-7351. Retrieved 2017-10-14.
- ^ 张忠海, 姜晓娟, 库宗军. 高氯酸镨与L-脯氨酸配合物的合成及热化学和热分解动力学研究[C]// 中国化学会全国化学热力学和热分析学术会议. 2006.

| |
| Identifiers | |
|---|---|
3D model (
JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.033.460 |
| EC Number |
|
PubChem
CID
|
|
| UN number | 3098 [2] |
| |
| Properties | |
| Pr(ClO4)3 | |
| Molar mass | 439.259 [1] |
| Density | 1.563 [2] |
| Melting point | liquid at room temperature [2] |
| Vapor pressure | 0.21 psi (20 °C) [2] |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Praseodymium(III) perchlorate is the perchlorate salt of praseodymium, with the chemical formula of Pr(ClO4)3. [3]
Preparation
Praseodymium(III) perchlorate can be prepared from praseodymium(III,IV) oxide. [4] [5] Dissolving praseodymium(III,IV) oxide in a slight excess of hydrochloric acid and adding a small amount of hydrogen peroxide can prepare praseodymium perchlorate. [5]
Chemical properties
Praseodymium perchlorate can form two complexes with crown ether 18-crown-6 in stoichiometric ratios of 1:1 and 1:2., [6] and can form complexes with L- proline, [7] glutamic acid, [4] mandelic acid, [4] penicillamine. [4] It can also form complexes with imidazole and alanine. [5]
References
- ^ a b c "Praseodymium perchlorate". ChemSpider. Retrieved 2017-10-21.
- ^ a b c d e "Praseodymium perchlorate 13498-07-2". www.chemicalbook.com. Retrieved 2017-10-20.
- ^ PubChem. "Praseodymium(3+) perchlorate". pubchem.ncbi.nlm.nih.gov. Retrieved 2022-07-11.
- ^ a b c d 张若桦, 陈敬堂、江冬青 (1995). "镨(III)溶液光谱研究:高氯酸镨─谷氨酸/苦杏仁酸/青霉胺配合物". Acta Scientiarum Naturalium Universitatis Nankaiensis (1): 44-48. ISSN 0465-7942. Retrieved 2017-10-21.
- ^ a b c 但悠梦, 胡卫兵、余华光、董家新、刘义、屈松生 (2006). "稀土配合物[Pr(C3H7NO2)2(C3H4N2)(H2O)](ClO4)3的标准生成焓及热分解动力学研究" [Standard Molar Enthalpy of Formation and Thermal Decomposition Kinetic Study of Rare Earth Complex [Pr(C3H7NO2)2(C3H4N2)(H2O)](ClO4)3]. Acta Chimica Sinica. 64 (1): 70-78. doi: 10.3321/j.issn:0567-7351.2006.01.012. ISSN 0567-7351. S2CID 236111702. Retrieved 2017-10-21.
- ^ Xue Ganglin; Li Qianding; Hu Shaoming; Xu Hong; Ren Dehou; Zhuang Quanzhen (1996). "三水合高氯酸镨与18—冠—6配合物的研究" [Study on complexes of trihydrated praseodymium perchlorate with 18 -crown-6]. Acta Chimica Sinica (in Chinese). 54 (6): 568-574. ISSN 0567-7351. Retrieved 2017-10-14.
- ^ 张忠海, 姜晓娟, 库宗军. 高氯酸镨与L-脯氨酸配合物的合成及热化学和热分解动力学研究[C]// 中国化学会全国化学热力学和热分析学术会议. 2006.