 | |
| Clinical data | |
|---|---|
| AHFS/ Drugs.com | Micromedex Detailed Consumer Information |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard ( EPA) | |
| ECHA InfoCard | 100.020.543 |
| Chemical and physical data | |
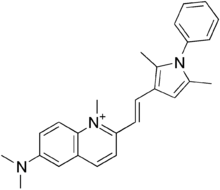
| Formula | C26H28N3+ |
| Molar mass | 382.531 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
Pyrvinium (Viprynium) is an anthelmintic effective for pinworms. [1] Several forms of pyrvinium have been prepared with variable counter anions, such as halides, tosylate, triflate and pamoate. [2] [3] Pyrvinium was identified as a potent Wnt inhibitor, acting through activation of Casein kinase CK1α. [4] [5]
Pyrvinium salts can also inhibit the growth of cancer cells. [6] More specifically, the pamoate salt has been shown to have preferential toxicity for various cancer cell lines during glucose starvation. [7]
Synthesis
One synthetic method is based on Skraup synthesis and Paal-Knorr synthesis. [6] More recently, an alternative convergent, synthetic strategy to pyrvinium triflate salts through Friedländer synthesis was reported. [3]
References
- ^ Desai AS (December 1962). "Single-dose treatment of oxyuriasis with pyrvinium embonate". British Medical Journal. 2 (5319): 1583–5. doi: 10.1136/bmj.2.5319.1583. PMC 1926864. PMID 14027194.
- ^ "Pyrvinium". PubChem. U.S. National Library of Medicine.
- ^
a
b Mao Y, Lin N, Tian W, Huang Z (2012). "New Synthesis of Pyrvinium That inhibits the β-Catenin/Tcf4 Pathway". Heterocycles. 85 (5): 1179–1185.
doi:
10.3987/COM-12-12446 (inactive 2024-02-17).
{{ cite journal}}: CS1 maint: DOI inactive as of February 2024 ( link) -
^ Saraswati S, Alfaro MP, Thorne CA, Atkinson J, Lee E, Young PP (2010).
"Pyrvinium, a potent small molecule Wnt inhibitor, promotes wound repair and post-MI cardiac remodeling". PLOS ONE. 5 (11): e15521.
Bibcode:
2010PLoSO...515521S.
doi:
10.1371/journal.pone.0015521.
PMC
2993965.
PMID
21170416.
 This article incorporates text from this source, which is available under the
CC0 license.
This article incorporates text from this source, which is available under the
CC0 license.
- ^ Shen C, Nayak A, Melendez RA, Robbins DJ (2020). "Casein Kinase 1α as a Regulator of Wnt-Driven Cancer". International Journal of Molecular Sciences. 21 (16): 5940. doi: 10.3390/ijms21165940. PMC 7460588. PMID 32824859.
- ^ a b WO 2006078754, Macdonald JE, Hysell MK, Yu D, Li H, Wong-Staal F, "Novel Quinolinium Salts and Derivatives", published 2006-07-27
- ^ Esumi H, Lu J, Kurashima Y, Hanaoka T (August 2004). "Antitumor activity of pyrvinium pamoate, 6-(dimethylamino)-2-[2-(2,5-dimethyl-1-phenyl-1H-pyrrol-3-yl)ethenyl]-1-methyl-quinolinium pamoate salt, showing preferential cytotoxicity during glucose starvation". Cancer Science. 95 (8): 685–90. doi: 10.1111/j.1349-7006.2004.tb03330.x. PMC 11159109. PMID 15298733.
 | |
| Clinical data | |
|---|---|
| AHFS/ Drugs.com | Micromedex Detailed Consumer Information |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard ( EPA) | |
| ECHA InfoCard | 100.020.543 |
| Chemical and physical data | |
| Formula | C26H28N3+ |
| Molar mass | 382.531 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
Pyrvinium (Viprynium) is an anthelmintic effective for pinworms. [1] Several forms of pyrvinium have been prepared with variable counter anions, such as halides, tosylate, triflate and pamoate. [2] [3] Pyrvinium was identified as a potent Wnt inhibitor, acting through activation of Casein kinase CK1α. [4] [5]
Pyrvinium salts can also inhibit the growth of cancer cells. [6] More specifically, the pamoate salt has been shown to have preferential toxicity for various cancer cell lines during glucose starvation. [7]
Synthesis
One synthetic method is based on Skraup synthesis and Paal-Knorr synthesis. [6] More recently, an alternative convergent, synthetic strategy to pyrvinium triflate salts through Friedländer synthesis was reported. [3]
References
- ^ Desai AS (December 1962). "Single-dose treatment of oxyuriasis with pyrvinium embonate". British Medical Journal. 2 (5319): 1583–5. doi: 10.1136/bmj.2.5319.1583. PMC 1926864. PMID 14027194.
- ^ "Pyrvinium". PubChem. U.S. National Library of Medicine.
- ^
a
b Mao Y, Lin N, Tian W, Huang Z (2012). "New Synthesis of Pyrvinium That inhibits the β-Catenin/Tcf4 Pathway". Heterocycles. 85 (5): 1179–1185.
doi:
10.3987/COM-12-12446 (inactive 2024-02-17).
{{ cite journal}}: CS1 maint: DOI inactive as of February 2024 ( link) -
^ Saraswati S, Alfaro MP, Thorne CA, Atkinson J, Lee E, Young PP (2010).
"Pyrvinium, a potent small molecule Wnt inhibitor, promotes wound repair and post-MI cardiac remodeling". PLOS ONE. 5 (11): e15521.
Bibcode:
2010PLoSO...515521S.
doi:
10.1371/journal.pone.0015521.
PMC
2993965.
PMID
21170416.
 This article incorporates text from this source, which is available under the
CC0 license.
This article incorporates text from this source, which is available under the
CC0 license.
- ^ Shen C, Nayak A, Melendez RA, Robbins DJ (2020). "Casein Kinase 1α as a Regulator of Wnt-Driven Cancer". International Journal of Molecular Sciences. 21 (16): 5940. doi: 10.3390/ijms21165940. PMC 7460588. PMID 32824859.
- ^ a b WO 2006078754, Macdonald JE, Hysell MK, Yu D, Li H, Wong-Staal F, "Novel Quinolinium Salts and Derivatives", published 2006-07-27
- ^ Esumi H, Lu J, Kurashima Y, Hanaoka T (August 2004). "Antitumor activity of pyrvinium pamoate, 6-(dimethylamino)-2-[2-(2,5-dimethyl-1-phenyl-1H-pyrrol-3-yl)ethenyl]-1-methyl-quinolinium pamoate salt, showing preferential cytotoxicity during glucose starvation". Cancer Science. 95 (8): 685–90. doi: 10.1111/j.1349-7006.2004.tb03330.x. PMC 11159109. PMID 15298733.