| Ubiquitin—protein ligase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 6.3.2.19 | ||||||||
| CAS no. | 74812-49-0 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
| Ubiquitin-conjugating enzyme, E2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | UBQ-conjugat_E2 | ||||||||
| Pfam | PF00179 | ||||||||
| InterPro | IPR000608 | ||||||||
| SMART | SM00212 | ||||||||
| PROSITE | PDOC00163 | ||||||||
| Membranome | 241 | ||||||||
| |||||||||
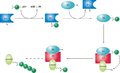
Ubiquitin-conjugating enzymes, also known as E2 enzymes and more rarely as ubiquitin-carrier enzymes, perform the second step in the ubiquitination reaction that targets a protein for degradation via the proteasome. The ubiquitination process covalently attaches ubiquitin, a short protein of 76 amino acids, to a lysine residue on the target protein. Once a protein has been tagged with one ubiquitin molecule, additional rounds of ubiquitination form a polyubiquitin chain that is recognized by the proteasome's 19S regulatory particle, triggering the ATP-dependent unfolding of the target protein that allows passage into the proteasome's 20S core particle, where proteases degrade the target into short peptide fragments for recycling by the cell. [1]
Relationships
A ubiquitin-activating enzyme, or E1, first activates the ubiquitin by covalently attaching the molecule to its active site cysteine residue. The activated ubiquitin is then transferred to an E2 cysteine. Once conjugated to ubiquitin, the E2 molecule binds one of several ubiquitin ligases or E3s via a structurally conserved binding region. The E3 molecule is responsible for binding the target protein substrate and transferring the ubiquitin from the E2 cysteine to a lysine residue on the target protein. [1]
-
Schematic diagram of the ubiquitylation system.
A particular cell usually contains only a few types of E1 molecule, a greater diversity of E2s, and a very large variety of E3s. In humans, there are about 30 E2s which can bind with one of the 600+ E3s. [2] The E3 molecules responsible for substrate identification and binding are thus the mechanisms of substrate specificity in proteasomal degradation. Each type of E2 can associate with many E3s. [3]
E2s can also be used to study protein folding mechanisms. Since the ubiquitylation system is shared across all organisms, studies can use modified E2 proteins in order to understand the overall system for how all organisms process proteins. [4] There are also some proteins which can act as both and E2 and an E3 containing domains which cover both E2 and E3 functionality. [5]
Isozymes
The following human genes encode ubiquitin-conjugating enzymes:
- UBE2A
- UBE2B
- UBE2C
- UBE2D1, UBE2D2, UBE2D3, UBE2D4 (the latter putative)
- UBE2E1, UBE2E2, UBE2E3
- UBE2F (putative)
- UBE2G1, UBE2G2
- UBE2H
- UBE2I
- UBE2J1, UBE2J2
- UBE2K
- UBE2L3, UBE2L6; ( UBE2L1, UBE2L2, UBE2L4 are pseudogenes)
- UBE2M
- UBE2N
- UBE2O
- UBE2Q1, UBE2Q2
- UBE2R1 (CDC34), UBE2R2
- UBE2S
- UBE2T (putative)
- UBE2U (putative)
- UBE2V1, UBE2V2
- UBE2W (putative)
- UBE2Z
- ATG3
- BIRC6
- UFC1
See also
References
- ^ a b Nandi D, Tahiliani P, Kumar A, Chandu D (March 2006). "The ubiquitin-proteasome system". Journal of Biosciences. 31 (1): 137–155. doi: 10.1007/BF02705243. PMID 16595883. S2CID 21603835.
- ^ Burge RJ, Damianou A, Wilkinson AJ, Rodenko B, Mottram JC (October 2020). "Leishmania differentiation requires ubiquitin conjugation mediated by a UBC2-UEV1 E2 complex". PLOS Pathogens. 16 (10): e1008784. doi: 10.1371/journal.ppat.1008784. PMC 7647121. PMID 33108402.
- ^ Risseeuw EP, Daskalchuk TE, Banks TW, Liu E, Cotelesage J, Hellmann H, et al. (June 2003). "Protein interaction analysis of SCF ubiquitin E3 ligase subunits from Arabidopsis". The Plant Journal. 34 (6): 753–767. doi: 10.1046/j.1365-313X.2003.01768.x. PMID 12795696.
- ^ Henneberg LT, Schulman BA (July 2021). "Decoding the messaging of the ubiquitin system using chemical and protein probes". Cell Chemical Biology. 28 (7): 889–902. doi: 10.1016/j.chembiol.2021.03.009. PMC 7611516. PMID 33831368.
- ^ Chang SC, Zhang BX, Ding JL (March 2022). "E2-E3 ubiquitin enzyme pairing - partnership in provoking or mitigating cancers". Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 1877 (2): 188679. doi: 10.1016/j.bbcan.2022.188679. PMID 35074437. S2CID 246224363.
External links
- Eukaryotic Linear Motif resource motif class MOD_SUMO
- Ubiquitin-Conjugating+Enzymes at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
| Ubiquitin—protein ligase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 6.3.2.19 | ||||||||
| CAS no. | 74812-49-0 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
| Ubiquitin-conjugating enzyme, E2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | UBQ-conjugat_E2 | ||||||||
| Pfam | PF00179 | ||||||||
| InterPro | IPR000608 | ||||||||
| SMART | SM00212 | ||||||||
| PROSITE | PDOC00163 | ||||||||
| Membranome | 241 | ||||||||
| |||||||||
Ubiquitin-conjugating enzymes, also known as E2 enzymes and more rarely as ubiquitin-carrier enzymes, perform the second step in the ubiquitination reaction that targets a protein for degradation via the proteasome. The ubiquitination process covalently attaches ubiquitin, a short protein of 76 amino acids, to a lysine residue on the target protein. Once a protein has been tagged with one ubiquitin molecule, additional rounds of ubiquitination form a polyubiquitin chain that is recognized by the proteasome's 19S regulatory particle, triggering the ATP-dependent unfolding of the target protein that allows passage into the proteasome's 20S core particle, where proteases degrade the target into short peptide fragments for recycling by the cell. [1]
Relationships
A ubiquitin-activating enzyme, or E1, first activates the ubiquitin by covalently attaching the molecule to its active site cysteine residue. The activated ubiquitin is then transferred to an E2 cysteine. Once conjugated to ubiquitin, the E2 molecule binds one of several ubiquitin ligases or E3s via a structurally conserved binding region. The E3 molecule is responsible for binding the target protein substrate and transferring the ubiquitin from the E2 cysteine to a lysine residue on the target protein. [1]
-
Schematic diagram of the ubiquitylation system.
A particular cell usually contains only a few types of E1 molecule, a greater diversity of E2s, and a very large variety of E3s. In humans, there are about 30 E2s which can bind with one of the 600+ E3s. [2] The E3 molecules responsible for substrate identification and binding are thus the mechanisms of substrate specificity in proteasomal degradation. Each type of E2 can associate with many E3s. [3]
E2s can also be used to study protein folding mechanisms. Since the ubiquitylation system is shared across all organisms, studies can use modified E2 proteins in order to understand the overall system for how all organisms process proteins. [4] There are also some proteins which can act as both and E2 and an E3 containing domains which cover both E2 and E3 functionality. [5]
Isozymes
The following human genes encode ubiquitin-conjugating enzymes:
- UBE2A
- UBE2B
- UBE2C
- UBE2D1, UBE2D2, UBE2D3, UBE2D4 (the latter putative)
- UBE2E1, UBE2E2, UBE2E3
- UBE2F (putative)
- UBE2G1, UBE2G2
- UBE2H
- UBE2I
- UBE2J1, UBE2J2
- UBE2K
- UBE2L3, UBE2L6; ( UBE2L1, UBE2L2, UBE2L4 are pseudogenes)
- UBE2M
- UBE2N
- UBE2O
- UBE2Q1, UBE2Q2
- UBE2R1 (CDC34), UBE2R2
- UBE2S
- UBE2T (putative)
- UBE2U (putative)
- UBE2V1, UBE2V2
- UBE2W (putative)
- UBE2Z
- ATG3
- BIRC6
- UFC1
See also
References
- ^ a b Nandi D, Tahiliani P, Kumar A, Chandu D (March 2006). "The ubiquitin-proteasome system". Journal of Biosciences. 31 (1): 137–155. doi: 10.1007/BF02705243. PMID 16595883. S2CID 21603835.
- ^ Burge RJ, Damianou A, Wilkinson AJ, Rodenko B, Mottram JC (October 2020). "Leishmania differentiation requires ubiquitin conjugation mediated by a UBC2-UEV1 E2 complex". PLOS Pathogens. 16 (10): e1008784. doi: 10.1371/journal.ppat.1008784. PMC 7647121. PMID 33108402.
- ^ Risseeuw EP, Daskalchuk TE, Banks TW, Liu E, Cotelesage J, Hellmann H, et al. (June 2003). "Protein interaction analysis of SCF ubiquitin E3 ligase subunits from Arabidopsis". The Plant Journal. 34 (6): 753–767. doi: 10.1046/j.1365-313X.2003.01768.x. PMID 12795696.
- ^ Henneberg LT, Schulman BA (July 2021). "Decoding the messaging of the ubiquitin system using chemical and protein probes". Cell Chemical Biology. 28 (7): 889–902. doi: 10.1016/j.chembiol.2021.03.009. PMC 7611516. PMID 33831368.
- ^ Chang SC, Zhang BX, Ding JL (March 2022). "E2-E3 ubiquitin enzyme pairing - partnership in provoking or mitigating cancers". Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 1877 (2): 188679. doi: 10.1016/j.bbcan.2022.188679. PMID 35074437. S2CID 246224363.
External links
- Eukaryotic Linear Motif resource motif class MOD_SUMO
- Ubiquitin-Conjugating+Enzymes at the U.S. National Library of Medicine Medical Subject Headings (MeSH)