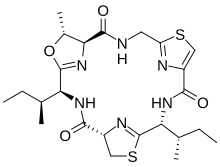
Cyclamides are a class of oligopeptides produced by cyanobacteria algae strains such as Microcystis aeruginosa. Some of them can be toxic.
Cyclamides are cyclopeptides with either six or eight amino acids, some of which are modified from their natural proteinogenic form. [1] They are typically characterized by thiazole and oxazole rings which are thought to be cysteine and threonine derivatives, respectively. [1] Cyclamides are biosynthesized through ribosomic pathways. [2] [3]
See also
References
- ^ a b "Cyclamides – a wealth of different bioactivities". NaToxAq Toxin of the week. NaToxAq, University of Copenhagen. 29 October 2018.
- ^ Ramsy Agha; Samuel Cirés; Lars Wörmer & Antonio Quesada (2013). "Limited Stability of Microcystins in Oligopeptide Compositions of Microcystis aeruginosa (Cyanobacteria): Implications in the Definition of Chemotypes". Toxins. 5 (6): 1089–1104. doi: 10.3390/toxins5061089. PMC 3717771. PMID 23744054.
- ^ Cyril Portmann; Judith F. Blom; Karl Gademann & Friedrich Jüttner (2008). "Aerucyclamides A and B: Isolation and Synthesis of Toxic Ribosomal Heterocyclic Peptides from the Cyanobacterium Microcystis aeruginosa PCC 7806". J. Nat. Prod. 71 (7): 1193–6. doi: 10.1021/np800118g. PMID 18558743.
External links
- "Cyanobacteria Are Far From Just Toledo's Problem". The New York Times. August 7, 2014.

Cyclamides are a class of oligopeptides produced by cyanobacteria algae strains such as Microcystis aeruginosa. Some of them can be toxic.
Cyclamides are cyclopeptides with either six or eight amino acids, some of which are modified from their natural proteinogenic form. [1] They are typically characterized by thiazole and oxazole rings which are thought to be cysteine and threonine derivatives, respectively. [1] Cyclamides are biosynthesized through ribosomic pathways. [2] [3]
See also
References
- ^ a b "Cyclamides – a wealth of different bioactivities". NaToxAq Toxin of the week. NaToxAq, University of Copenhagen. 29 October 2018.
- ^ Ramsy Agha; Samuel Cirés; Lars Wörmer & Antonio Quesada (2013). "Limited Stability of Microcystins in Oligopeptide Compositions of Microcystis aeruginosa (Cyanobacteria): Implications in the Definition of Chemotypes". Toxins. 5 (6): 1089–1104. doi: 10.3390/toxins5061089. PMC 3717771. PMID 23744054.
- ^ Cyril Portmann; Judith F. Blom; Karl Gademann & Friedrich Jüttner (2008). "Aerucyclamides A and B: Isolation and Synthesis of Toxic Ribosomal Heterocyclic Peptides from the Cyanobacterium Microcystis aeruginosa PCC 7806". J. Nat. Prod. 71 (7): 1193–6. doi: 10.1021/np800118g. PMID 18558743.
External links
- "Cyanobacteria Are Far From Just Toledo's Problem". The New York Times. August 7, 2014.