→Side effects: Clarified the CARET/ATBC dosing regimen. |
|||
| Line 69: | Line 69: | ||
==External links== |
==External links== |
||
* Goji beta-carotene sources, [http://www.agoji.com/ Goji Beta-Carotene] |
|||
* {{ATC|A11|CA02}} |
* {{ATC|A11|CA02}} |
||
*[http://www.ars.usda.gov/research/publications/publications.htm?SEQ_NO_115=153965 USDA Webpage on β-carotene Content of Gac] - Fatty Acids and Carotenoids in Gac (Momordica Cochinchinensis Spreng) Fruit. |
*[http://www.ars.usda.gov/research/publications/publications.htm?SEQ_NO_115=153965 USDA Webpage on β-carotene Content of Gac] - Fatty Acids and Carotenoids in Gac (Momordica Cochinchinensis Spreng) Fruit. |
||
Revision as of 22:49, 13 June 2009

| |
| |
| Names | |
|---|---|
|
IUPAC name
β,β-carotene
| |
| Identifiers | |
3D model (
JSmol)
|
|
| ECHA InfoCard | 100.027.851 |
| E number | E160a (colours) |
PubChem
CID
|
|
CompTox Dashboard (
EPA)
|
|
| |
| Properties | |
| C40H56 | |
| Molar mass | 536.87 g/mol |
| Appearance | red-purple solid |
| Density | 0.941 ± 0.06 g/cm3 |
| Melting point | 180-182 °C |
| Insoluble in cold water or hot water. Soluble in diethyl ether, acetone, benzene, chloroform, carbon disulfide. Moderately soluble in petroleium ether, oils.Very slightly soluble in methanol.
Soluble in fat solvents | |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
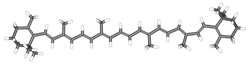
β-Carotene is an organic compound - a terpenoid, a red-orange pigment abundant in plants and fruits. As a carotene with β-rings at both ends, it is the most common form of carotene. It is a precursor (inactive form) of vitamin A. [1] Being highly conjugated, it is deeply colored, and as a hydrocarbon lacking functional groups, it is very lipophilic.
The structure was deduced by Karrer et al. [2] In nature, β-carotene is a precursor to vitamin A via the action of beta-carotene 15,15'-monooxygenase. β-Carotene is also the substance in carrots that colours them orange. β-Carotenoid is biosynthesized from geranylgeranyl pyrophosphate. [1]
Pro-vitamin A activity and sources in the diet
Plant carotenoids are the primary dietary source of pro-vitamin A worldwide. The most efficient pro-vitamin A carotenoid is β-carotene, which is abundant in Vietnam Gac (Momordica Cochinchinensis Spreng), crude palm oil, yellow and orange fruits, such as mangoes and papayas, orange root vegetables such as carrots and yams and in green leafy vegetables such as spinach, kale, sweet potato leaves, and sweet gourd leaves. Vietnam gac and crude palm oil have by far the highest content of β-carotene of any known fruit or vegetable, 10 times higher than carrots for example. However, Gac is quite rare and unknown outside its native region of SE Asia, and crude palm oil is typically processed to remove the cartenoids before sale to improve the color and clarity.
Side effects
The most common side effect of excessive β-carotene consumption is carotenodermia, a harmless condition that presents as a conspicuous orange skin tint arising from deposition of the carotenoid in the outermost layer of the epidermis [3]. Chronic, high doses of β-carotene have been associated with increased rate of lung cancer among those who smoke. [4]
See also
References
- ^
a
b Susan D. Van Arnum (1998). "Vitamin A in Kirk-Othmer Encyclopedia of Chemical Technology" (45). New York: John Wiley: 99–107.
doi:
10.1002/0471238961.2209200101181421.a01.
{{ cite journal}}: Cite journal requires|journal=( help) -
^ P. Karrer, A. Helfenstein, H. Wehrli, A. Wettstein (1930). "Pflanzenfarbstoffe XXV. Über die Konstitution des Lycopins und Carotins".
Helvetica Chimica Acta. 13: 1084–1099.
doi:
10.1002/hlca.19300130532.
{{ cite journal}}: CS1 maint: multiple names: authors list ( link) -
^ Stahl W, Heinrich U, Jungmann H; et al. (1998). "Increased Dermal Carotenoid Levels Assessed by Noninvasive Reflection Spectrophotometry Correlate with Serum Levels in Women Ingesting Betatene". Journal of Nutrition. 128 (5): 903–7.
PMID
9567001.
{{ cite journal}}: Explicit use of et al. in:|author=( help)CS1 maint: multiple names: authors list ( link) -
^ Tanvetyanon T, Bepler G (2008). "Beta-carotene in multivitamins and the possible risk of lung cancer among smokers versus former smokers: a meta-analysis and evaluation of national brands". Cancer. 113 (1): 150–7.
doi:
10.1002/cncr.23527.
PMID
18429004.
{{ cite journal}}: Unknown parameter|month=ignored ( help)
External links
- Goji beta-carotene sources, Goji Beta-Carotene
- A11CA02 ( WHO)
- USDA Webpage on β-carotene Content of Gac - Fatty Acids and Carotenoids in Gac (Momordica Cochinchinensis Spreng) Fruit.
→Side effects: Clarified the CARET/ATBC dosing regimen. |
|||
| Line 69: | Line 69: | ||
==External links== |
==External links== |
||
* Goji beta-carotene sources, [http://www.agoji.com/ Goji Beta-Carotene] |
|||
* {{ATC|A11|CA02}} |
* {{ATC|A11|CA02}} |
||
*[http://www.ars.usda.gov/research/publications/publications.htm?SEQ_NO_115=153965 USDA Webpage on β-carotene Content of Gac] - Fatty Acids and Carotenoids in Gac (Momordica Cochinchinensis Spreng) Fruit. |
*[http://www.ars.usda.gov/research/publications/publications.htm?SEQ_NO_115=153965 USDA Webpage on β-carotene Content of Gac] - Fatty Acids and Carotenoids in Gac (Momordica Cochinchinensis Spreng) Fruit. |
||
Revision as of 22:49, 13 June 2009

| |

| |
| Names | |
|---|---|
|
IUPAC name
β,β-carotene
| |
| Identifiers | |
3D model (
JSmol)
|
|
| ECHA InfoCard | 100.027.851 |
| E number | E160a (colours) |
PubChem
CID
|
|
CompTox Dashboard (
EPA)
|
|
| |
| Properties | |
| C40H56 | |
| Molar mass | 536.87 g/mol |
| Appearance | red-purple solid |
| Density | 0.941 ± 0.06 g/cm3 |
| Melting point | 180-182 °C |
| Insoluble in cold water or hot water. Soluble in diethyl ether, acetone, benzene, chloroform, carbon disulfide. Moderately soluble in petroleium ether, oils.Very slightly soluble in methanol.
Soluble in fat solvents | |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
β-Carotene is an organic compound - a terpenoid, a red-orange pigment abundant in plants and fruits. As a carotene with β-rings at both ends, it is the most common form of carotene. It is a precursor (inactive form) of vitamin A. [1] Being highly conjugated, it is deeply colored, and as a hydrocarbon lacking functional groups, it is very lipophilic.
The structure was deduced by Karrer et al. [2] In nature, β-carotene is a precursor to vitamin A via the action of beta-carotene 15,15'-monooxygenase. β-Carotene is also the substance in carrots that colours them orange. β-Carotenoid is biosynthesized from geranylgeranyl pyrophosphate. [1]
Pro-vitamin A activity and sources in the diet
Plant carotenoids are the primary dietary source of pro-vitamin A worldwide. The most efficient pro-vitamin A carotenoid is β-carotene, which is abundant in Vietnam Gac (Momordica Cochinchinensis Spreng), crude palm oil, yellow and orange fruits, such as mangoes and papayas, orange root vegetables such as carrots and yams and in green leafy vegetables such as spinach, kale, sweet potato leaves, and sweet gourd leaves. Vietnam gac and crude palm oil have by far the highest content of β-carotene of any known fruit or vegetable, 10 times higher than carrots for example. However, Gac is quite rare and unknown outside its native region of SE Asia, and crude palm oil is typically processed to remove the cartenoids before sale to improve the color and clarity.
Side effects
The most common side effect of excessive β-carotene consumption is carotenodermia, a harmless condition that presents as a conspicuous orange skin tint arising from deposition of the carotenoid in the outermost layer of the epidermis [3]. Chronic, high doses of β-carotene have been associated with increased rate of lung cancer among those who smoke. [4]
See also
References
- ^
a
b Susan D. Van Arnum (1998). "Vitamin A in Kirk-Othmer Encyclopedia of Chemical Technology" (45). New York: John Wiley: 99–107.
doi:
10.1002/0471238961.2209200101181421.a01.
{{ cite journal}}: Cite journal requires|journal=( help) -
^ P. Karrer, A. Helfenstein, H. Wehrli, A. Wettstein (1930). "Pflanzenfarbstoffe XXV. Über die Konstitution des Lycopins und Carotins".
Helvetica Chimica Acta. 13: 1084–1099.
doi:
10.1002/hlca.19300130532.
{{ cite journal}}: CS1 maint: multiple names: authors list ( link) -
^ Stahl W, Heinrich U, Jungmann H; et al. (1998). "Increased Dermal Carotenoid Levels Assessed by Noninvasive Reflection Spectrophotometry Correlate with Serum Levels in Women Ingesting Betatene". Journal of Nutrition. 128 (5): 903–7.
PMID
9567001.
{{ cite journal}}: Explicit use of et al. in:|author=( help)CS1 maint: multiple names: authors list ( link) -
^ Tanvetyanon T, Bepler G (2008). "Beta-carotene in multivitamins and the possible risk of lung cancer among smokers versus former smokers: a meta-analysis and evaluation of national brands". Cancer. 113 (1): 150–7.
doi:
10.1002/cncr.23527.
PMID
18429004.
{{ cite journal}}: Unknown parameter|month=ignored ( help)
External links
- Goji beta-carotene sources, Goji Beta-Carotene
- A11CA02 ( WHO)
- USDA Webpage on β-carotene Content of Gac - Fatty Acids and Carotenoids in Gac (Momordica Cochinchinensis Spreng) Fruit.