| |
| Names | |
|---|---|
|
Preferred IUPAC name
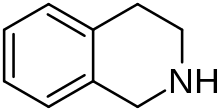
1,2,3,4-Tetrahydroisoquinoline | |
| Identifiers | |
3D model (
JSmol)
|
|
| Abbreviations | TIQ, THIQ |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.864 |
| EC Number |
|
PubChem
CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C9H11N | |
| Molar mass | 133.19 g/mol |
| Appearance | Deep yellow liquid |
| Density | 1.05 g/mL |
| Melting point | −30 °C (−22 °F; 243 K) |
| Boiling point | 235 to 239 °C (455 to 462 °F; 508 to 512 K) |
| Hazards | |
| GHS labelling: [1] | |



| |
| Danger | |
| H301, H310, H314, H332, H371, H412 | |
| P260, P261, P262, P264, P270, P271, P273, P280, P301+P310, P301+P330+P331, P302+P350, P302+P352, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P309+P311, P310, P312, P322, P330, P332+P313, P337+P313, P361, P362, P363, P403+P233, P405, P501 | |
| Flash point | 99 °C (210 °F; 372 K) (closed cup) |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Tetrahydroisoquinoline (TIQ or THIQ) is an organic compound with the chemical formula C9H11N. Classified as a secondary amine, it is derived from isoquinoline by hydrogenation. It is a colorless viscous liquid that is miscible with most organic solvents. The tetrahydroisoquinoline skeleton is encountered in a number of bioactive compounds and drugs. [2] [3]
As a secondary amine, tetrahydroisoquinoline has weakly basic properties and forms salts with strong acids. It can be dehydrogenated to give isoquinoline and hydrogenated to decahydroisoquinoline. Like other secondary amines, tetrahydroisoquinoline can be oxidized to the corresponding nitrone using hydrogen peroxide, catalyzed by selenium dioxide. [4]
Tetrahydroisoquinoline derivatives may be formed in the body as metabolites of some drugs, and this was once thought to be involved in the development of alcoholism. [5] This theory has now been discredited and is no longer generally accepted by the scientific community, [6] but endogenous production of neurotoxic tetrahydroisoquinoline derivatives such as norsalsolinol continue to be investigated as possible causes for some conditions such as Parkinson's disease. [7] [8] [9] [10] [11] [12]
The tetrahydroisoquinoline skeleton is present in a number of drugs, [3] such as tubocurarine, one of the quaternary ammonium muscle relaxants. Drugs based on 4-substituted tetrahydroisoquinolines include nomifensine [13] and diclofensine. They can be prepared by N-alkylation of benzyl amines with halo acetophenones. [14] Naturally occurring tetrahydroisoquinolines include cherylline [15] and latifine.
Esproquin, [16] which shows hypotensive activity by virtue of its α-adrenergic blocking properties, is made from THIQ.
- ^ "1,2,3,4-Tetrahydroisoquinoline". pubchem.ncbi.nlm.nih.gov. Retrieved 12 December 2021.
- ^ Mitchenson, Andrew (2000). "Saturated nitrogen heterocycles". Journal of the Chemical Society, Perkin Transactions 1 (17): 2862–2892. doi: 10.1039/A908537H.
- ^ a b Scott, Jack D.; Williams, Robert M. (2002). "Chemistry and Biology of the Tetrahydroisoquinoline Antitumor Antibiotics". Chemical Reviews. 102 (5): 1669–1730. doi: 10.1021/cr010212u. PMID 11996547.
- ^ Murahashi, S. (1987). "Selenium dioxide catalyzed oxidation of secondary amines with hydrogen peroxide. Simple synthesis of nitrones from secondary amines". Tetrahedron Letters. 28 (21): 2383–2386. doi: 10.1016/S0040-4039(00)96130-6.
- ^ Blum, K.; Hamilton, M. G.; Hirst, M.; Wallace, J. E. (1978). "Putative role of isoquinoline alkaloids in alcoholism: a link to opiates". Alcoholism: Clinical and Experimental Research. 2 (2): 113–120. doi: 10.1111/j.1530-0277.1978.tb04710.x. PMID 350073.,Altshuler, H. L.; Shippenberg (1982). "Tetrahydroisoquinoline and opioid substrates of alcohol actions". Progress in Clinical and Biological Research. 90: 329–344. PMID 7202207., Myers, R. D. (1989). "Isoquinolines, beta-carbolines and alcohol drinking: involvement of opioid and dopaminergic mechanisms". Experientia. 45 (5): 436–443. doi: 10.1007/BF01952025. PMID 2656285. S2CID 1513683.
- ^ Myers, R. D. (1996). "Tetrahydroisoquinolines and alcoholism: where are we today?". Alcoholism: Clinical and Experimental Research. 20 (3): 498–500. doi: 10.1111/j.1530-0277.1996.tb01081.x. PMID 8727243., Musshoff, F.; Daldrup, T.; Bonte, W.; Leitner, A.; Lesch, O. M. (1996). "Formaldehyde-derived tetrahydroisoquinolines and tetrahydro-beta-carbolines in human urine". Journal of Chromatography B. 683 (2): 163–176. doi: 10.1016/0378-4347(96)00106-5. PMID 8891913., Sällström Baum, S.; Hill, R.; Kiianmaa, K.; Rommelspacher, H. (1999). "Effect of ethanol on (R)- and (S)-salsolinol, salsoline, and THP in the nucleus accumbens of AA and ANA rats". Alcohol (Fayetteville, N.Y.). 18 (2–3): 165–169. doi: 10.1016/S0741-8329(98)00080-9. PMID 10456568., Musshoff, F.; Lachenmeier, D. W.; Schmidt, P.; Dettmeyer, R.; Madea, B. (2005). "Systematic regional study of dopamine, norsalsolinol, and (R/S)-salsolinol levels in human brain areas of alcoholics". Alcoholism: Clinical and Experimental Research. 29 (1): 46–52. doi: 10.1097/01.ALC.0000150011.81102.C2. PMID 15654290.
- ^ Kotake Y, Tasaki Y, Makino Y, Ohta S, Hirobe M (December 1995). "1-Benzyl-1,2,3,4-tetrahydroisoquinoline as a parkinsonism-inducing agent: a novel endogenous amine in mouse brain and parkinsonian CSF". Journal of Neurochemistry. 65 (6): 2633–8. doi: 10.1046/j.1471-4159.1995.65062633.x. PMID 7595560. S2CID 39449026.
- ^ McNaught KS, Carrupt PA, Altomare C, Cellamare S, Carotti A, Testa B, Jenner P, Marsden CD (October 1998). "Isoquinoline derivatives as endogenous neurotoxins in the aetiology of Parkinson's disease". Biochemical Pharmacology. 56 (8): 921–33. doi: 10.1016/S0006-2952(98)00142-7. PMID 9776302.
- ^ Lorenc-Koci E, Smiałowska M, Antkiewicz-Michaluk L, Gołembiowska K, Bajkowska M, Wolfarth S (2000). "Effect of acute and chronic administration of 1,2,3,4-tetrahydroisoquinoline on muscle tone, metabolism of dopamine in the striatum and tyrosine hydroxylase immunocytochemistry in the substantia nigra, in rats". Neuroscience. 95 (4): 1049–59. doi: 10.1016/S0306-4522(99)00511-4. PMID 10682712. S2CID 13549697.
- ^ Storch A, Ott S, Hwang YI, Ortmann R, Hein A, Frenzel S, Matsubara K, Ohta S, Wolf HU, Schwarz J (March 2002). "Selective dopaminergic neurotoxicity of isoquinoline derivatives related to Parkinson's disease: studies using heterologous expression systems of the dopamine transporter". Biochemical Pharmacology. 63 (5): 909–20. doi: 10.1016/S0006-2952(01)00922-4. PMID 11911843.
- ^ Lorenc-Koci E, Antkiewicz-Michaluk L, Kamińska A, Lenda T, Zieba B, Wierońska J, Smiałowska M, Schulze G, Rommelspacher H (October 2008). "The influence of acute and chronic administration of 1,2-dimethyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline on the function of the nigrostriatal dopaminergic system in rats". Neuroscience. 156 (4): 973–86. doi: 10.1016/j.neuroscience.2008.08.050. PMID 18809471. S2CID 44658852.
- ^ Kobayashi H, Fukuhara K, Tada-Oikawa S, Yada Y, Hiraku Y, Murata M, Oikawa S (January 2009). "The mechanisms of oxidative DNA damage and apoptosis induced by norsalsolinol, an endogenous tetrahydroisoquinoline derivative associated with Parkinson's disease". Journal of Neurochemistry. 108 (2): 397–407. doi: 10.1111/j.1471-4159.2008.05774.x. PMID 19012744.
- ^ Schneider, C. S.; Weber, K. H.; Daniel, H.; Bechtel, W. D.; Boeke-Kuhn, K. (1984). "Synthesis and antidepressant activity of 4-aryltetrahydrothieno[2,3-c]pyridine derivatives". Journal of Medicinal Chemistry. 27 (9): 1150–1155. doi: 10.1021/jm00375a011. PMID 6471069.
- ^ BG 49761
- ^ cherylline
- ^ Gray, Allan P.; Shiley, Richard H. (1973). "Preparation and cardiovascular actions of a group of tetrahydroisoquinoline derivatives". Journal of Medicinal Chemistry. 16 (7): 859–861. doi: 10.1021/jm00265a028. ISSN 0022-2623. PMID 4146907.

| |
| Names | |
|---|---|
|
Preferred IUPAC name
1,2,3,4-Tetrahydroisoquinoline | |
| Identifiers | |
3D model (
JSmol)
|
|
| Abbreviations | TIQ, THIQ |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.864 |
| EC Number |
|
PubChem
CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C9H11N | |
| Molar mass | 133.19 g/mol |
| Appearance | Deep yellow liquid |
| Density | 1.05 g/mL |
| Melting point | −30 °C (−22 °F; 243 K) |
| Boiling point | 235 to 239 °C (455 to 462 °F; 508 to 512 K) |
| Hazards | |
| GHS labelling: [1] | |



| |
| Danger | |
| H301, H310, H314, H332, H371, H412 | |
| P260, P261, P262, P264, P270, P271, P273, P280, P301+P310, P301+P330+P331, P302+P350, P302+P352, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P309+P311, P310, P312, P322, P330, P332+P313, P337+P313, P361, P362, P363, P403+P233, P405, P501 | |
| Flash point | 99 °C (210 °F; 372 K) (closed cup) |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Tetrahydroisoquinoline (TIQ or THIQ) is an organic compound with the chemical formula C9H11N. Classified as a secondary amine, it is derived from isoquinoline by hydrogenation. It is a colorless viscous liquid that is miscible with most organic solvents. The tetrahydroisoquinoline skeleton is encountered in a number of bioactive compounds and drugs. [2] [3]
As a secondary amine, tetrahydroisoquinoline has weakly basic properties and forms salts with strong acids. It can be dehydrogenated to give isoquinoline and hydrogenated to decahydroisoquinoline. Like other secondary amines, tetrahydroisoquinoline can be oxidized to the corresponding nitrone using hydrogen peroxide, catalyzed by selenium dioxide. [4]
Tetrahydroisoquinoline derivatives may be formed in the body as metabolites of some drugs, and this was once thought to be involved in the development of alcoholism. [5] This theory has now been discredited and is no longer generally accepted by the scientific community, [6] but endogenous production of neurotoxic tetrahydroisoquinoline derivatives such as norsalsolinol continue to be investigated as possible causes for some conditions such as Parkinson's disease. [7] [8] [9] [10] [11] [12]
The tetrahydroisoquinoline skeleton is present in a number of drugs, [3] such as tubocurarine, one of the quaternary ammonium muscle relaxants. Drugs based on 4-substituted tetrahydroisoquinolines include nomifensine [13] and diclofensine. They can be prepared by N-alkylation of benzyl amines with halo acetophenones. [14] Naturally occurring tetrahydroisoquinolines include cherylline [15] and latifine.
Esproquin, [16] which shows hypotensive activity by virtue of its α-adrenergic blocking properties, is made from THIQ.
- ^ "1,2,3,4-Tetrahydroisoquinoline". pubchem.ncbi.nlm.nih.gov. Retrieved 12 December 2021.
- ^ Mitchenson, Andrew (2000). "Saturated nitrogen heterocycles". Journal of the Chemical Society, Perkin Transactions 1 (17): 2862–2892. doi: 10.1039/A908537H.
- ^ a b Scott, Jack D.; Williams, Robert M. (2002). "Chemistry and Biology of the Tetrahydroisoquinoline Antitumor Antibiotics". Chemical Reviews. 102 (5): 1669–1730. doi: 10.1021/cr010212u. PMID 11996547.
- ^ Murahashi, S. (1987). "Selenium dioxide catalyzed oxidation of secondary amines with hydrogen peroxide. Simple synthesis of nitrones from secondary amines". Tetrahedron Letters. 28 (21): 2383–2386. doi: 10.1016/S0040-4039(00)96130-6.
- ^ Blum, K.; Hamilton, M. G.; Hirst, M.; Wallace, J. E. (1978). "Putative role of isoquinoline alkaloids in alcoholism: a link to opiates". Alcoholism: Clinical and Experimental Research. 2 (2): 113–120. doi: 10.1111/j.1530-0277.1978.tb04710.x. PMID 350073.,Altshuler, H. L.; Shippenberg (1982). "Tetrahydroisoquinoline and opioid substrates of alcohol actions". Progress in Clinical and Biological Research. 90: 329–344. PMID 7202207., Myers, R. D. (1989). "Isoquinolines, beta-carbolines and alcohol drinking: involvement of opioid and dopaminergic mechanisms". Experientia. 45 (5): 436–443. doi: 10.1007/BF01952025. PMID 2656285. S2CID 1513683.
- ^ Myers, R. D. (1996). "Tetrahydroisoquinolines and alcoholism: where are we today?". Alcoholism: Clinical and Experimental Research. 20 (3): 498–500. doi: 10.1111/j.1530-0277.1996.tb01081.x. PMID 8727243., Musshoff, F.; Daldrup, T.; Bonte, W.; Leitner, A.; Lesch, O. M. (1996). "Formaldehyde-derived tetrahydroisoquinolines and tetrahydro-beta-carbolines in human urine". Journal of Chromatography B. 683 (2): 163–176. doi: 10.1016/0378-4347(96)00106-5. PMID 8891913., Sällström Baum, S.; Hill, R.; Kiianmaa, K.; Rommelspacher, H. (1999). "Effect of ethanol on (R)- and (S)-salsolinol, salsoline, and THP in the nucleus accumbens of AA and ANA rats". Alcohol (Fayetteville, N.Y.). 18 (2–3): 165–169. doi: 10.1016/S0741-8329(98)00080-9. PMID 10456568., Musshoff, F.; Lachenmeier, D. W.; Schmidt, P.; Dettmeyer, R.; Madea, B. (2005). "Systematic regional study of dopamine, norsalsolinol, and (R/S)-salsolinol levels in human brain areas of alcoholics". Alcoholism: Clinical and Experimental Research. 29 (1): 46–52. doi: 10.1097/01.ALC.0000150011.81102.C2. PMID 15654290.
- ^ Kotake Y, Tasaki Y, Makino Y, Ohta S, Hirobe M (December 1995). "1-Benzyl-1,2,3,4-tetrahydroisoquinoline as a parkinsonism-inducing agent: a novel endogenous amine in mouse brain and parkinsonian CSF". Journal of Neurochemistry. 65 (6): 2633–8. doi: 10.1046/j.1471-4159.1995.65062633.x. PMID 7595560. S2CID 39449026.
- ^ McNaught KS, Carrupt PA, Altomare C, Cellamare S, Carotti A, Testa B, Jenner P, Marsden CD (October 1998). "Isoquinoline derivatives as endogenous neurotoxins in the aetiology of Parkinson's disease". Biochemical Pharmacology. 56 (8): 921–33. doi: 10.1016/S0006-2952(98)00142-7. PMID 9776302.
- ^ Lorenc-Koci E, Smiałowska M, Antkiewicz-Michaluk L, Gołembiowska K, Bajkowska M, Wolfarth S (2000). "Effect of acute and chronic administration of 1,2,3,4-tetrahydroisoquinoline on muscle tone, metabolism of dopamine in the striatum and tyrosine hydroxylase immunocytochemistry in the substantia nigra, in rats". Neuroscience. 95 (4): 1049–59. doi: 10.1016/S0306-4522(99)00511-4. PMID 10682712. S2CID 13549697.
- ^ Storch A, Ott S, Hwang YI, Ortmann R, Hein A, Frenzel S, Matsubara K, Ohta S, Wolf HU, Schwarz J (March 2002). "Selective dopaminergic neurotoxicity of isoquinoline derivatives related to Parkinson's disease: studies using heterologous expression systems of the dopamine transporter". Biochemical Pharmacology. 63 (5): 909–20. doi: 10.1016/S0006-2952(01)00922-4. PMID 11911843.
- ^ Lorenc-Koci E, Antkiewicz-Michaluk L, Kamińska A, Lenda T, Zieba B, Wierońska J, Smiałowska M, Schulze G, Rommelspacher H (October 2008). "The influence of acute and chronic administration of 1,2-dimethyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline on the function of the nigrostriatal dopaminergic system in rats". Neuroscience. 156 (4): 973–86. doi: 10.1016/j.neuroscience.2008.08.050. PMID 18809471. S2CID 44658852.
- ^ Kobayashi H, Fukuhara K, Tada-Oikawa S, Yada Y, Hiraku Y, Murata M, Oikawa S (January 2009). "The mechanisms of oxidative DNA damage and apoptosis induced by norsalsolinol, an endogenous tetrahydroisoquinoline derivative associated with Parkinson's disease". Journal of Neurochemistry. 108 (2): 397–407. doi: 10.1111/j.1471-4159.2008.05774.x. PMID 19012744.
- ^ Schneider, C. S.; Weber, K. H.; Daniel, H.; Bechtel, W. D.; Boeke-Kuhn, K. (1984). "Synthesis and antidepressant activity of 4-aryltetrahydrothieno[2,3-c]pyridine derivatives". Journal of Medicinal Chemistry. 27 (9): 1150–1155. doi: 10.1021/jm00375a011. PMID 6471069.
- ^ BG 49761
- ^ cherylline
- ^ Gray, Allan P.; Shiley, Richard H. (1973). "Preparation and cardiovascular actions of a group of tetrahydroisoquinoline derivatives". Journal of Medicinal Chemistry. 16 (7): 859–861. doi: 10.1021/jm00265a028. ISSN 0022-2623. PMID 4146907.