
In organic chemistry, a nitrone is a functional group consisting of an N-oxide of an imine. The general structure is R1R2C=N+(−O−)(−R3), where R3 is not a hydrogen. Their primary application is intermediates in chemical synthesis. A nitrone is a 1,3-dipole used in cycloadditions, and a carbonyl mimic.
Nitrones, as a tetrasubstituted double bond, admit cis–trans isomerism. [1]: 474
Typical nitrone sources are hydroxylamine oxidation or condensation with carbonyl compounds. Secondary hydroxylamines oxidize to nitrones in air over a timescale of several weeks, a process cupric salts accelerate. [1]: 476 [2]: 332–333 The most general reagent used for the oxidation of hydroxylamines is aqueous mercuric oxide: [1]: 476 [3]
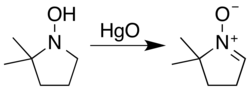
However, a hydroxylamine with two α hydrogens may unsaturate on either side. Carbonyl condensation avoids this ambiguity... [4]

...but is inhibited if both ketone substituents are bulky. [1]: 477
In principle, N- alkylation could produce nitrones from oximes, but in practice electrophiles typically perform a mixture of N- and O-attack. [1]: 479 [2]: 334
Some nitrones oligomerize: [1]: 483 [2]: 334,337-338 [5]
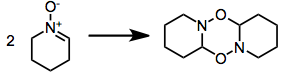
Syntheses with nitrone precursors obviate the issue with increased temperature, to exaggerate entropic factors; or with a nitrone excess.
Like many other unsaturated functional groups, nitrones activate the α and β carbons towards reaction. The α carbon is an electrophile and the β carbon a nucleophile; that is, nitrones polarize like carbonyls and nitriles but unlike nitro compounds and vinyl sulfur derivatives. [1]: 483 [2]: 338–340
Nitrones hydrolyze extremely easily to the corresponding carbonyl and N-hydroxylamine. [1]: 491 [2]: 344
As 1,3‑dipoles, nitrones perform [3+2] cycloadditions. [6] For example, a dipolarophilic alkene combines to form isoxazolidine:

Other ring-closing reactions are known, [7] including formal [3+3] and [5+2] cycloadditions. [6]
Deoxygenating reagents, light, or heat all catalyze rearrangement to the amide. Acids catalyze rearrangement to the oxime ether. [1]: 489–490 [2]: 345–347
Hydrides add to give hydroxylamines. Reducing Lewis acids (e.g. metals, SO2) deoxygenate to the imine instead. [1]: 490 [2]: 343
- ^ a b c d e f g h i j Hamer, Jan; Macaluso, Anthony (1964-08-01). "Nitrones". Chemical Reviews. 64 (4): 473–495. doi: 10.1021/cr60230a006. ISSN 0009-2665.
- ^ a b c d e f g Delpierre, G. R.; Lamchen, M. (1965). "Nitrones". Quarterly Reviews, Chemical Society. 19 (4): 329. doi: 10.1039/qr9651900329. ISSN 0009-2681.
- ^ Thiesing, Jan; Mayer, Hans (1957). "Cyclische Nitrone, II. Über die Polymeren des 2.3.4.5-Tetrahydro-pyridin-N-oxyds und verwandte Verbindungen". Justus Liebigs Ann. Chem. 609: 46-57. doi: 10.1002/jlac.19576090105.
- ^ Exner, O. (1951). "A New Synthesis of N-methylketoximes". ChemPlusChem. 16: 258-267. doi: 10.1135/cccc19510258.
- ^ Thiesing, Jan; Mayer, Hans (1956). "Cyclische Nitrone I: Dimeres 2.3.4.5-Tetrahydro-pyridin-N-oxyd". Chem. Ber. 89 (9): 2159-2167. doi: 10.1002/cber.19560890919.
- ^ a b Yang, Jiong (2012). "Recent Developments in Nitrone Chemistry". Synlett. 23: 2293-97. doi: 10.1055/s-0032-1317096.
- ^ Murahashi, Shun-Ichi; Imada, Yasushi (15 March 2019). "Synthesis and Transformations of Nitrones for Organic Synthesis". Chemical Reviews. 119 (7): 4684–4716. doi: 10.1021/acs.chemrev.8b00476. PMID 30875202. S2CID 80623450.

In organic chemistry, a nitrone is a functional group consisting of an N-oxide of an imine. The general structure is R1R2C=N+(−O−)(−R3), where R3 is not a hydrogen. Their primary application is intermediates in chemical synthesis. A nitrone is a 1,3-dipole used in cycloadditions, and a carbonyl mimic.
Nitrones, as a tetrasubstituted double bond, admit cis–trans isomerism. [1]: 474
Typical nitrone sources are hydroxylamine oxidation or condensation with carbonyl compounds. Secondary hydroxylamines oxidize to nitrones in air over a timescale of several weeks, a process cupric salts accelerate. [1]: 476 [2]: 332–333 The most general reagent used for the oxidation of hydroxylamines is aqueous mercuric oxide: [1]: 476 [3]

However, a hydroxylamine with two α hydrogens may unsaturate on either side. Carbonyl condensation avoids this ambiguity... [4]

...but is inhibited if both ketone substituents are bulky. [1]: 477
In principle, N- alkylation could produce nitrones from oximes, but in practice electrophiles typically perform a mixture of N- and O-attack. [1]: 479 [2]: 334
Some nitrones oligomerize: [1]: 483 [2]: 334,337-338 [5]

Syntheses with nitrone precursors obviate the issue with increased temperature, to exaggerate entropic factors; or with a nitrone excess.
Like many other unsaturated functional groups, nitrones activate the α and β carbons towards reaction. The α carbon is an electrophile and the β carbon a nucleophile; that is, nitrones polarize like carbonyls and nitriles but unlike nitro compounds and vinyl sulfur derivatives. [1]: 483 [2]: 338–340
Nitrones hydrolyze extremely easily to the corresponding carbonyl and N-hydroxylamine. [1]: 491 [2]: 344
As 1,3‑dipoles, nitrones perform [3+2] cycloadditions. [6] For example, a dipolarophilic alkene combines to form isoxazolidine:

Other ring-closing reactions are known, [7] including formal [3+3] and [5+2] cycloadditions. [6]
Deoxygenating reagents, light, or heat all catalyze rearrangement to the amide. Acids catalyze rearrangement to the oxime ether. [1]: 489–490 [2]: 345–347
Hydrides add to give hydroxylamines. Reducing Lewis acids (e.g. metals, SO2) deoxygenate to the imine instead. [1]: 490 [2]: 343
- ^ a b c d e f g h i j Hamer, Jan; Macaluso, Anthony (1964-08-01). "Nitrones". Chemical Reviews. 64 (4): 473–495. doi: 10.1021/cr60230a006. ISSN 0009-2665.
- ^ a b c d e f g Delpierre, G. R.; Lamchen, M. (1965). "Nitrones". Quarterly Reviews, Chemical Society. 19 (4): 329. doi: 10.1039/qr9651900329. ISSN 0009-2681.
- ^ Thiesing, Jan; Mayer, Hans (1957). "Cyclische Nitrone, II. Über die Polymeren des 2.3.4.5-Tetrahydro-pyridin-N-oxyds und verwandte Verbindungen". Justus Liebigs Ann. Chem. 609: 46-57. doi: 10.1002/jlac.19576090105.
- ^ Exner, O. (1951). "A New Synthesis of N-methylketoximes". ChemPlusChem. 16: 258-267. doi: 10.1135/cccc19510258.
- ^ Thiesing, Jan; Mayer, Hans (1956). "Cyclische Nitrone I: Dimeres 2.3.4.5-Tetrahydro-pyridin-N-oxyd". Chem. Ber. 89 (9): 2159-2167. doi: 10.1002/cber.19560890919.
- ^ a b Yang, Jiong (2012). "Recent Developments in Nitrone Chemistry". Synlett. 23: 2293-97. doi: 10.1055/s-0032-1317096.
- ^ Murahashi, Shun-Ichi; Imada, Yasushi (15 March 2019). "Synthesis and Transformations of Nitrones for Organic Synthesis". Chemical Reviews. 119 (7): 4684–4716. doi: 10.1021/acs.chemrev.8b00476. PMID 30875202. S2CID 80623450.