| Square antiprismatic molecular geometry | |
|---|---|
 | |
| Examples |
XeF2− 8, ReF− 8 |
| Point group | D4d |
| Coordination number | 8 |
| μ (Polarity) | 0 |
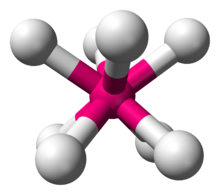
In chemistry, the square antiprismatic molecular geometry describes the shape of compounds where eight atoms, groups of atoms, or ligands are arranged around a central atom, defining the vertices of a square antiprism. [1] This shape has D4d symmetry and is one of the three common shapes for octacoordinate transition metal complexes, along with the dodecahedron and the bicapped trigonal prism. [2] [3]
Like with other high coordination numbers, eight-coordinate compounds are often distorted from idealized geometries, as illustrated by the structure of Na3TaF8. In this case, with the small Na+ ions, lattice forces are strong. With the diatomic cation NO+, the lattice forces are weaker, such as in (NO)2XeF8, which crystallizes with a more idealized square antiprismatic geometry.
-
The distorted square antiprismatic [TaF83− anion in the Na3TaF8 lattice. [4]
-
The square antiprismatic [XeF82− anion in the lattice of nitrosonium octafluoroxenate(VI), (NO)2XeF8. [5]
-
Structure of the Bi82+ cluster in the [Bi8](GaCl4)2.
Examples
-
XeF2−
8 - IF−
8 - ReF−
8
Square prismatic geometry and cubic geometry
Square prismatic geometry (D4h) is much less common compared to the square antiprism. An example of a molecular species with square prismatic geometry (a slightly flattened cube) is octafluoroprotactinate(V), [PaF83–, as found in its sodium salt, Na3PaF8. [6] While local cubic 8-coordination is common in ionic lattices (e.g., Ca2+ in CaF2), and some 8-coordinate actinide complexes are approximately cubic, there are no reported examples of rigorously cubic 8-coordinate molecular species. A number of other rare geometries for 8-coordination are also known. [2]
References
- ^ D. L. Kepert (1978). "Aspects of the Stereochemistry of Eight-Coordination". Progress in Inorganic Chemistry. 24: 179–249. doi: 10.1002/9780470166253.ch4. ISBN 9780470166253.
- ^ a b Jeremy K. Burdett; Roald Hoffmann; Robert C. Fay (1978). "Eight-Coordination". Inorganic Chemistry. 17 (9): 2553–2568. doi: 10.1021/ic50187a041.
- ^ Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6
- ^ Langer, V.; Smrčok, L.; Boča, M. (2010). "Redetermination of Na3TaF8". Acta Crystallographica Section C. 66 (9): pi85–pi86. doi: 10.1107/S0108270110030556. PMID 20814090.
- ^ Peterson, W.; Holloway, H.; Coyle, A.; Williams, M. (Sep 1971). "Antiprismatic Coordination about Xenon: the Structure of Nitrosonium Octafluoroxenate(VI)". Science. 173 (4003): 1238–1239. Bibcode: 1971Sci...173.1238P. doi: 10.1126/science.173.4003.1238. ISSN 0036-8075. PMID 17775218. S2CID 22384146.
- ^ Brown, D.; Easey, J. F.; Rickard, C. E. F. (1969). "Cubic co-ordination: crystal structure of sodium octafluoroprotactinate(V)". Journal of the Chemical Society A: Inorganic, Physical, Theoretical: 1161. doi: 10.1039/j19690001161. ISSN 0022-4944.
| Square antiprismatic molecular geometry | |
|---|---|
 | |
| Examples |
XeF2− 8, ReF− 8 |
| Point group | D4d |
| Coordination number | 8 |
| μ (Polarity) | 0 |
In chemistry, the square antiprismatic molecular geometry describes the shape of compounds where eight atoms, groups of atoms, or ligands are arranged around a central atom, defining the vertices of a square antiprism. [1] This shape has D4d symmetry and is one of the three common shapes for octacoordinate transition metal complexes, along with the dodecahedron and the bicapped trigonal prism. [2] [3]
Like with other high coordination numbers, eight-coordinate compounds are often distorted from idealized geometries, as illustrated by the structure of Na3TaF8. In this case, with the small Na+ ions, lattice forces are strong. With the diatomic cation NO+, the lattice forces are weaker, such as in (NO)2XeF8, which crystallizes with a more idealized square antiprismatic geometry.
-
The distorted square antiprismatic [TaF83− anion in the Na3TaF8 lattice. [4]
-
The square antiprismatic [XeF82− anion in the lattice of nitrosonium octafluoroxenate(VI), (NO)2XeF8. [5]
-
Structure of the Bi82+ cluster in the [Bi8](GaCl4)2.
Examples
-
XeF2−
8 - IF−
8 - ReF−
8
Square prismatic geometry and cubic geometry
Square prismatic geometry (D4h) is much less common compared to the square antiprism. An example of a molecular species with square prismatic geometry (a slightly flattened cube) is octafluoroprotactinate(V), [PaF83–, as found in its sodium salt, Na3PaF8. [6] While local cubic 8-coordination is common in ionic lattices (e.g., Ca2+ in CaF2), and some 8-coordinate actinide complexes are approximately cubic, there are no reported examples of rigorously cubic 8-coordinate molecular species. A number of other rare geometries for 8-coordination are also known. [2]
References
- ^ D. L. Kepert (1978). "Aspects of the Stereochemistry of Eight-Coordination". Progress in Inorganic Chemistry. 24: 179–249. doi: 10.1002/9780470166253.ch4. ISBN 9780470166253.
- ^ a b Jeremy K. Burdett; Roald Hoffmann; Robert C. Fay (1978). "Eight-Coordination". Inorganic Chemistry. 17 (9): 2553–2568. doi: 10.1021/ic50187a041.
- ^ Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6
- ^ Langer, V.; Smrčok, L.; Boča, M. (2010). "Redetermination of Na3TaF8". Acta Crystallographica Section C. 66 (9): pi85–pi86. doi: 10.1107/S0108270110030556. PMID 20814090.
- ^ Peterson, W.; Holloway, H.; Coyle, A.; Williams, M. (Sep 1971). "Antiprismatic Coordination about Xenon: the Structure of Nitrosonium Octafluoroxenate(VI)". Science. 173 (4003): 1238–1239. Bibcode: 1971Sci...173.1238P. doi: 10.1126/science.173.4003.1238. ISSN 0036-8075. PMID 17775218. S2CID 22384146.
- ^ Brown, D.; Easey, J. F.; Rickard, C. E. F. (1969). "Cubic co-ordination: crystal structure of sodium octafluoroprotactinate(V)". Journal of the Chemical Society A: Inorganic, Physical, Theoretical: 1161. doi: 10.1039/j19690001161. ISSN 0022-4944.
![The distorted square antiprismatic [TaF8]3− anion in the Na3TaF8 lattice.[4]](https://upload.wikimedia.org/wikipedia/commons/thumb/0/02/TaF83-Core.png/130px-TaF83-Core.png)
![The square antiprismatic [XeF8]2− anion in the lattice of nitrosonium octafluoroxenate(VI), (NO)2XeF8.[5]](https://upload.wikimedia.org/wikipedia/commons/thumb/3/30/Octafluoroxenate%28VI%29-3D-balls-A.png/164px-Octafluoroxenate%28VI%29-3D-balls-A.png)
2.](https://upload.wikimedia.org/wikipedia/commons/thumb/6/65/EntryWithCollCode414090.png/135px-EntryWithCollCode414090.png)