| Dodecahedral molecular geometry | |
|---|---|
 | |
| Examples | Mo(CN)4− 8 |
| Point group | D2d |
| Coordination number | 8 |
| μ (Polarity) | 0 |
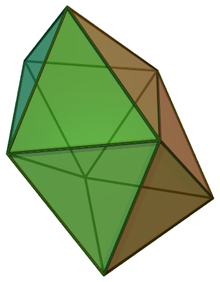
In chemistry, the dodecahedral molecular geometry describes the shape of compounds where eight atoms or groups of atoms or ligands are arranged around a central atom defining the vertices of a snub disphenoid (also known as a trigonal dodecahedron). This shape has D2d symmetry and is one of the three common shapes for octacoordinate transition metal complexes, along with the square antiprism and the bicapped trigonal prism. [1] [2]
One example of the dodecahedral molecular geometry is the Mo(CN)4−
8 ion.
[2]
References
- ^ Jeremy K. Burdett; Roald Hoffmann; Robert C. Fay (1978). "Eight-Coordination". Inorganic Chemistry. 17 (9): 2553–2568. doi: 10.1021/ic50187a041.
- ^ a b Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6
| Dodecahedral molecular geometry | |
|---|---|
 | |
| Examples | Mo(CN)4− 8 |
| Point group | D2d |
| Coordination number | 8 |
| μ (Polarity) | 0 |
In chemistry, the dodecahedral molecular geometry describes the shape of compounds where eight atoms or groups of atoms or ligands are arranged around a central atom defining the vertices of a snub disphenoid (also known as a trigonal dodecahedron). This shape has D2d symmetry and is one of the three common shapes for octacoordinate transition metal complexes, along with the square antiprism and the bicapped trigonal prism. [1] [2]
One example of the dodecahedral molecular geometry is the Mo(CN)4−
8 ion.
[2]
References
- ^ Jeremy K. Burdett; Roald Hoffmann; Robert C. Fay (1978). "Eight-Coordination". Inorganic Chemistry. 17 (9): 2553–2568. doi: 10.1021/ic50187a041.
- ^ a b Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6