In chemistry, paratungstate refers to the anion with the formula [W12O4212- and salts derived from this anion. The term also refers to protonated derivatives of this anion, including [H2W12O4210-. Ammonium paratungstate (or APT), (NH4)10[H2W12O42] is a key intermediate in the purification of tungsten from its ores. [1]
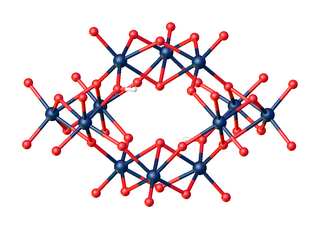
The salt (NH4)10(W12O42)·4H2O has been characterized by X-ray crystallography. [2]
The unprotonated anion [W12O4212- has C2h symmetry.
See also
- Metatungstate [W12O408-, with idealized Td symmetry.
References
- ^ Lassner, Erick; Schubert, Wolf-Dieter; Lüderitz, Eberhard; Wolf, Hans Uwe. "Tungsten, Tungsten Alloys, and Tungsten Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi: 10.1002/14356007.a27_229. ISBN 978-3527306732.
- ^ d'Amour, Hedwig; Allmann, Rudolf (1972). "Die Kristallstruktur des Ammoniumparawolframat-tetrahydrats (NH4)10[H2W12O42]·4H2O". Zeitschrift für Kristallographie. 136 (1–2): 23–47. Bibcode: 1972ZK....136...23D. doi: 10.1524/zkri.1972.136.1-2.23.
In chemistry, paratungstate refers to the anion with the formula [W12O4212- and salts derived from this anion. The term also refers to protonated derivatives of this anion, including [H2W12O4210-. Ammonium paratungstate (or APT), (NH4)10[H2W12O42] is a key intermediate in the purification of tungsten from its ores. [1]

The salt (NH4)10(W12O42)·4H2O has been characterized by X-ray crystallography. [2]
The unprotonated anion [W12O4212- has C2h symmetry.
See also
- Metatungstate [W12O408-, with idealized Td symmetry.
References
- ^ Lassner, Erick; Schubert, Wolf-Dieter; Lüderitz, Eberhard; Wolf, Hans Uwe. "Tungsten, Tungsten Alloys, and Tungsten Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi: 10.1002/14356007.a27_229. ISBN 978-3527306732.
- ^ d'Amour, Hedwig; Allmann, Rudolf (1972). "Die Kristallstruktur des Ammoniumparawolframat-tetrahydrats (NH4)10[H2W12O42]·4H2O". Zeitschrift für Kristallographie. 136 (1–2): 23–47. Bibcode: 1972ZK....136...23D. doi: 10.1524/zkri.1972.136.1-2.23.