| Cunninghamella echinulata | |
|---|---|
| |
|
Scientific classification
| |
| Domain: | Eukaryota |
| Kingdom: | Fungi |
| Division: | Mucoromycota |
| Class: | Mucoromycetes |
| Order: | Mucorales |
| Family: | Cunninghamellaceae |
| Genus: | Cunninghamella |
| Species: | C. echinulata
|
| Binomial name | |
| Cunninghamella echinulata | |
| Subspecies | |
|
Cunninghamella echinulata var. antarctica
| |
| Synonyms | |
| |
Cunninghamella echinulata is a fungal species in the genus Cunninghamella. [1] It is an asexually reproducing fungus and a mesophile, preferring intermediate temperature ranges. [1] [2] C. echinulata is a common air contaminant, [3] and is currently of interest to the biotechnology industry due to its ability to synthesize γ-linolenic acid [4] as well as its capacity to bioconcentrate metals. [5] This species is a soil saprotroph that forms rhizoids, [3] preferring soils enriched in nitrogen, phosphorus and potassium. [2] It has been reported occasionally an agent of mucormycosis following the inhalation of fungal spores. [6] Czapek's agar is a suitable growth medium for the propagation of C. echinulata. [7]
Taxonomy, growth and morphology
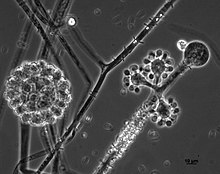
Cunninghamella echinulata is a member of the family, Cunninghamellaceae (phylum Mucoromycota). [1] [8] This species is closely related to C. elegans, and both species share highly similar characteristics of growth and morphology. Colonies tend to be rapidly growing on most growth media producing a dense, white or greyish aerial mycelium. [9] Cunninghamella echinulata reproduces asexually and solely via yellow-brown, spiny, single-spored sporangioles that, due to the nature of the sporangiospore being retained within the sporangium, appear to have a two-layered outer wall. [1] [10] [11] This fungus grows by means of filaments that lack septa. [6] [12] This is a common feature of members if the Mucoromycota where the hyphal compartments are either fully divided by septa or are completely continuous ( coenocytic) and multinucleate. Zygospores of this fungus are only produced following the fusion of gametangia of compatible mating strains, exemplifying a heterothallic mating system. [13] Sporangiophores of this species are irregularly branched and do not resemble the sporangiospores typical of most other members of the Mucoromycota encountered in similar habitats. [13] [9] The sporangioles produced by this fungus are larger in size (10–20 μm) than those of the closely related species, C. elegans. [13]
Physiology
Cunninghamella echinulata and other species of Cunninghamellaceae can be selectively grown on Czapek's solution agar, [7] a property unique to this family of the Mucorales. However, depending on the nutrients the agar is supplemented with, different media can alter the oxidative metabolism profile of this fungus. [14] This species grows better on acetate than d-glucose. [2] Additionally, if grown in liquid, cultures of this fungus can be externally stimulated to increase oxygen consumption by adding 2% montmorillonite or kaolinite. [2]
While this fungus is a mesophilic (preferring intermediate growth temperatures), it is able to grow between 6 °C (43 °F) and 45 °C (113 °F) although the rate of growth near the extremes of temperature tolerance is minimal. [2] [6] The optimal temperature for the development of zygospores is between 25 °C (77 °F) and 35 °C (95 °F). [2] This species exhibits different growth characteristic depending on environmental influences. At a pH of 5.5, the fungus grows in small dense pellets; [14] but a more typical, radiating growth pattern is achieved at a pH of 8.0, [14] The presence of indole-3-acetic acid in the growth medium stimulates linear growth. [2]
When grown on medium containing hydrolysed tomato residue, this fungus utilizes glucose to synthesize triacylglycerols (TAG) rich in GLA. [4] This fungus has been investigated for use in the production of single cell oils (SCO) and storage lipids (like GLA). [15] C. echinulata is also able to selectively take up and sequester metal contaminants from polluted waters, suggesting a potential use in bioremediation of polluted water. [5] However, its role as an agent of opportunistic disease may limit its use in environmental remediation. Cunninghamella echinulata is able to grow on orange rind and assimilate carbohydrates into necessary biomolecules, [15] where the fermented peel does not exhibit appreciable discolouration or odour. [15] Growth of this fungus on organic nitrogen leads yields lipids rich in γ-linolenic acid (GLA). [4] The presence of an active monooxygenase system allows this species to perform oxidative demethylation and hydroxylation. [2] The fungus possesses a p450 cytochrome system similar to that in humans, making it a potentially useful model for the study liver-mediated drug metabolism. [14]
This species is also able to stereoselectively biotransform rac-mexiletine into hydroxymethyl mexiletine (HMM) and p-hydroxymexiletine (PHM), two metabolites also produced in humans. [14] Cunninghamella echinulata grown in yeast extract broth, trypticase soy medium or peptone broth at a pH of 8 yielded 0 μg/ml of breakdown products from the metabolism of rac-mexiletine. [14] The production of maximal HMM is achieved in yeast extract broth at a pH of 7.0. [14] Metabolic activity diminishes with increasing pH up to a maximum pH of 8.0. [14] At increased pH, C. echinulata shows preferential production of S-HMM over R-HMM, the two stereoisomers, specifically enantiomers, of HMM. [14] In order to achieve the highest quantity of GLA, Cunninghamella echinulata grows preferentially on nitrogen-depleted media with a C/N (carbon:nitrogen) molar ratio of 169. [15]
The species has been reported to exhibit antibacterial effects against Staphylococcus aureus and Salmonella typhus, [2] common agents of skin infections and food poisoning respectively. It is also known to inhibit root growth in various grass species in vitro. [2] The fungus is not known to produce mycotoxins. [13]
Habitat and ecology
Cunninghamella echinulata is a saprotrophic resident of the soils in warmer regions of the world, particularly those enriched with NPK fertilizers (Nitrogen, Phosphorus and Potassium). [1] [2] It has been reported from both cultivated and uncultivated soils, [9] [16] including soils from greenhouses and forests [7] in the mediterranean and subtropical zones but is thought to be comparatively rare in temperate zones. [9] [13] Soil depth and pH are not considered to be strongly influential on the growth properties of this fungus in vivo. [2] This species is able to cause rot in foods such as Kola nuts [13] and is a common air contaminant. [3] It can be parasitized by other fungi including species of Piptocephalis, [12] and Trichoderma viride. [2] Additionally, its growth is inhibited in vitro by the fungus, Memnoniella echinata. [2]
Human disease
Disease caused by this fungus and other species of Mucorales is referred to as mucormycosis characterized by a rapidly progressive and destructively invasive disease with relatively low survival. [6] Literature reporting this agent in healthy people is lacking. As a consequence, this species is thought to be exclusively an opportunistic pathogen, affecting individuals with pre-existing health conditions. People with underlying health conditions such as HIV infection and diabetes are at heightened risk for mucormycosis. [6] Infections by C. echinulata are thought to arise from inhalation of fungal spores and are not communicable. [6] Relatively few case reports implicating C. echinulata are available. Of those that are, one prototypical case from 2005 reported a fatal rhinocerebral infection in a 15-year-old boy suffering from acute leukaemia. [6] Biopsy of the infected nasal tissue showed signs of necrosis and vascular invasion. [6]
Cunninghamella echinulata, like other members of the genus, exhibit strong resistance to the antifungal polyene, amphotericin B with a MIC (Minimum Inhibitory Concentration) ranging from 4-16μg/mL that varies according to strain. [6] Strains of C. echinulata also display greater tolerance to itraconazole and posaconazole than other members of the Mucorales. [6] The antifungal agent terbinafine, typically restricted to the treatment of nail and skin infections, shows a relatively low MIC ranging from 0.06 to 0.125 μg/mL. [6]
Biotechnology
It is commonly cultivated for its ability to produce GLA, [4] preferentially synthesizing R-PHM and S-HMM. [14] The fungus is able to synthesize γ-linolenic acid. [4] It also possesses the ability to bioabsorb metals, with the highest levels of bioabsorption reported 5 to 15 minutes after contact with the metals. [5] Adding NaOH to this fungus before it absorbs metals enhances the uptake of Pb, Cu and Zn. [5] These uptake rates also seem to be influenced by pH where at a pH of 7.1, Zn was the most highly absorbed metal, [5] at a pH of 4, Pb was the most highly absorbed metal [5] and at a pH of 5, Cu was the most highly absorbed metal. [5] Cunninghamella echinulata has been used to transform cortexolone to hydrocortisone. [17] Hydroxylation of biphenyl oxide has been studied in C. echinulata. [18]
References
- ^ a b c d e Webster, John (1980). Introduction to Fungi. Cambridge: Cambridge university press. pp. 230. ISBN 0-521-22888-3.
- ^
a
b
c
d
e
f
g
h
i
j
k
l
m
n Domsch, Gams, Heidi-Anderson, K.H, W, Traute (1980). Compendium of Soil Fungi (Vol I). Academic Press. p. 238.
{{ cite book}}: CS1 maint: multiple names: authors list ( link) - ^ a b c Skinner, Charles (1947). Molds, Yeasts and Actinomycetes. New York: John Wiley & Sons. p. 92.
- ^ a b c d e De-Wei, Li (2016). Biology of Microfungi. Springer. p. 555. ISBN 9783319291376.
- ^ a b c d e f g El-Morsy, El-Sayed M. (2016-12-01). "Cunninghamella echinulata a new biosorbent of metal ions from polluted water in Egypt". Mycologia. 96 (6): 1183–1189. doi: 10.2307/3762133. ISSN 0027-5514. JSTOR 3762133. PMID 21148940.
- ^ a b c d e f g h i j k LeBlanc, Robert E.; Meriden, Zina; Sutton, Deanna A.; Thompson, Elizabeth H.; Neofytos, Dionissios; Zhang, Sean X. (2013-08-01). "Cunninghamella echinulata causing fatally invasive fungal sinusitis". Diagnostic Microbiology and Infectious Disease. 76 (4): 506–509. doi: 10.1016/j.diagmicrobio.2013.03.009. ISSN 1879-0070. PMID 23602784.
- ^ a b c Mycology Guidebook. Seattle and London: University of Washington press. 1974. pp. 94, 96, 97. ISBN 0-295-95313-6.
- ^ Hawker, Lilian (1966). Fungi. London: Hutchinson University library. p. 76.
- ^ a b c d L. O.Donnell, Kerry (1979). Zygomycetes in Culture. Dept. of Botany, University of Georgia. p. 236.
- ^ Khan (1975). "Wall Structure and Germination of Spores in Cunninghamella echinulata". Journal of General Microbiology. 90: 115–124. doi: 10.1099/00221287-90-1-115.
- ^ Watanabe, Tsuneo (2010). Pictorial Atlas of Soil and Seed Fungi. New York: CRC press. p. 69.
- ^
a
b Gwynne-Vaughan, Barnes, H.C.I, B. (1927). Structure and Development of the Fungi. New York: The Macmillan company. pp. 115, 116.
{{ cite book}}: CS1 maint: multiple names: authors list ( link) - ^
a
b
c
d
e
f Pitt, Hocking, John, Ailsa (1999). Fungi and Food Spoilage (2nd ED). Springer. pp. 178–180.
ISBN
978-0-387-92206-5.
{{ cite book}}: CS1 maint: multiple names: authors list ( link) - ^ a b c d e f g h i j Freitag, D. G; Foster, R. T; Coutts, R. T; Pickard, M. A; Pasutto, F. M (1997). "Stereoselective metabolism of rac-mexiletine by the fungus Cunninghamella echinulata yields the major human metabolites hydroxymethylmexiletine and p-hydroxymexiletine". Drug Metab Dispos. 25 (6): 685–692. PMID 9193869.
- ^ a b c d Gema, H.; Kavadia, A.; Dimou, D.; Tsagou, V.; Komaitis, M.; Aggelis, G. (2002). "Production of γ-linolenic acid by Cunninghamella echinulata cultivated on glucose and orange peel". Applied Microbiology and Biotechnology. 58 (3): 303–307. doi: 10.1007/s00253-001-0910-7. ISSN 0175-7598. PMID 11935180. S2CID 2346913.
- ^ Dennis, R.W.G (1986). Fungi of the Hebrides. Kew: Royal Botanic Gardens. p. 231. ISBN 0-947643-02-8.
- ^ Manosroi, J.; Chisti, Y.; Manosroi, A. (2006). "Biotransformation of cortexolone to hydrocortisone by molds using a rapid color development assay". Prikladnaia Biokhimiia I Mikrobiologiia. 42 (5): 547–551. PMID 17066954.
- ^ Seigle-Murandi, F. M.; Krivobok, S. M. A.; Steiman, R. L.; Benoit-Guyod, J. L. A.; Thiault, G. A. (1991). "Biphenyl oxide hydroxylation by Cunninghamella echinulata". Journal of Agricultural and Food Chemistry. 39 (2): 428. doi: 10.1021/jf00002a041.
| Cunninghamella echinulata | |
|---|---|

| |
|
Scientific classification
| |
| Domain: | Eukaryota |
| Kingdom: | Fungi |
| Division: | Mucoromycota |
| Class: | Mucoromycetes |
| Order: | Mucorales |
| Family: | Cunninghamellaceae |
| Genus: | Cunninghamella |
| Species: | C. echinulata
|
| Binomial name | |
| Cunninghamella echinulata | |
| Subspecies | |
|
Cunninghamella echinulata var. antarctica
| |
| Synonyms | |
| |
Cunninghamella echinulata is a fungal species in the genus Cunninghamella. [1] It is an asexually reproducing fungus and a mesophile, preferring intermediate temperature ranges. [1] [2] C. echinulata is a common air contaminant, [3] and is currently of interest to the biotechnology industry due to its ability to synthesize γ-linolenic acid [4] as well as its capacity to bioconcentrate metals. [5] This species is a soil saprotroph that forms rhizoids, [3] preferring soils enriched in nitrogen, phosphorus and potassium. [2] It has been reported occasionally an agent of mucormycosis following the inhalation of fungal spores. [6] Czapek's agar is a suitable growth medium for the propagation of C. echinulata. [7]
Taxonomy, growth and morphology
Cunninghamella echinulata is a member of the family, Cunninghamellaceae (phylum Mucoromycota). [1] [8] This species is closely related to C. elegans, and both species share highly similar characteristics of growth and morphology. Colonies tend to be rapidly growing on most growth media producing a dense, white or greyish aerial mycelium. [9] Cunninghamella echinulata reproduces asexually and solely via yellow-brown, spiny, single-spored sporangioles that, due to the nature of the sporangiospore being retained within the sporangium, appear to have a two-layered outer wall. [1] [10] [11] This fungus grows by means of filaments that lack septa. [6] [12] This is a common feature of members if the Mucoromycota where the hyphal compartments are either fully divided by septa or are completely continuous ( coenocytic) and multinucleate. Zygospores of this fungus are only produced following the fusion of gametangia of compatible mating strains, exemplifying a heterothallic mating system. [13] Sporangiophores of this species are irregularly branched and do not resemble the sporangiospores typical of most other members of the Mucoromycota encountered in similar habitats. [13] [9] The sporangioles produced by this fungus are larger in size (10–20 μm) than those of the closely related species, C. elegans. [13]
Physiology
Cunninghamella echinulata and other species of Cunninghamellaceae can be selectively grown on Czapek's solution agar, [7] a property unique to this family of the Mucorales. However, depending on the nutrients the agar is supplemented with, different media can alter the oxidative metabolism profile of this fungus. [14] This species grows better on acetate than d-glucose. [2] Additionally, if grown in liquid, cultures of this fungus can be externally stimulated to increase oxygen consumption by adding 2% montmorillonite or kaolinite. [2]
While this fungus is a mesophilic (preferring intermediate growth temperatures), it is able to grow between 6 °C (43 °F) and 45 °C (113 °F) although the rate of growth near the extremes of temperature tolerance is minimal. [2] [6] The optimal temperature for the development of zygospores is between 25 °C (77 °F) and 35 °C (95 °F). [2] This species exhibits different growth characteristic depending on environmental influences. At a pH of 5.5, the fungus grows in small dense pellets; [14] but a more typical, radiating growth pattern is achieved at a pH of 8.0, [14] The presence of indole-3-acetic acid in the growth medium stimulates linear growth. [2]
When grown on medium containing hydrolysed tomato residue, this fungus utilizes glucose to synthesize triacylglycerols (TAG) rich in GLA. [4] This fungus has been investigated for use in the production of single cell oils (SCO) and storage lipids (like GLA). [15] C. echinulata is also able to selectively take up and sequester metal contaminants from polluted waters, suggesting a potential use in bioremediation of polluted water. [5] However, its role as an agent of opportunistic disease may limit its use in environmental remediation. Cunninghamella echinulata is able to grow on orange rind and assimilate carbohydrates into necessary biomolecules, [15] where the fermented peel does not exhibit appreciable discolouration or odour. [15] Growth of this fungus on organic nitrogen leads yields lipids rich in γ-linolenic acid (GLA). [4] The presence of an active monooxygenase system allows this species to perform oxidative demethylation and hydroxylation. [2] The fungus possesses a p450 cytochrome system similar to that in humans, making it a potentially useful model for the study liver-mediated drug metabolism. [14]
This species is also able to stereoselectively biotransform rac-mexiletine into hydroxymethyl mexiletine (HMM) and p-hydroxymexiletine (PHM), two metabolites also produced in humans. [14] Cunninghamella echinulata grown in yeast extract broth, trypticase soy medium or peptone broth at a pH of 8 yielded 0 μg/ml of breakdown products from the metabolism of rac-mexiletine. [14] The production of maximal HMM is achieved in yeast extract broth at a pH of 7.0. [14] Metabolic activity diminishes with increasing pH up to a maximum pH of 8.0. [14] At increased pH, C. echinulata shows preferential production of S-HMM over R-HMM, the two stereoisomers, specifically enantiomers, of HMM. [14] In order to achieve the highest quantity of GLA, Cunninghamella echinulata grows preferentially on nitrogen-depleted media with a C/N (carbon:nitrogen) molar ratio of 169. [15]
The species has been reported to exhibit antibacterial effects against Staphylococcus aureus and Salmonella typhus, [2] common agents of skin infections and food poisoning respectively. It is also known to inhibit root growth in various grass species in vitro. [2] The fungus is not known to produce mycotoxins. [13]
Habitat and ecology
Cunninghamella echinulata is a saprotrophic resident of the soils in warmer regions of the world, particularly those enriched with NPK fertilizers (Nitrogen, Phosphorus and Potassium). [1] [2] It has been reported from both cultivated and uncultivated soils, [9] [16] including soils from greenhouses and forests [7] in the mediterranean and subtropical zones but is thought to be comparatively rare in temperate zones. [9] [13] Soil depth and pH are not considered to be strongly influential on the growth properties of this fungus in vivo. [2] This species is able to cause rot in foods such as Kola nuts [13] and is a common air contaminant. [3] It can be parasitized by other fungi including species of Piptocephalis, [12] and Trichoderma viride. [2] Additionally, its growth is inhibited in vitro by the fungus, Memnoniella echinata. [2]
Human disease
Disease caused by this fungus and other species of Mucorales is referred to as mucormycosis characterized by a rapidly progressive and destructively invasive disease with relatively low survival. [6] Literature reporting this agent in healthy people is lacking. As a consequence, this species is thought to be exclusively an opportunistic pathogen, affecting individuals with pre-existing health conditions. People with underlying health conditions such as HIV infection and diabetes are at heightened risk for mucormycosis. [6] Infections by C. echinulata are thought to arise from inhalation of fungal spores and are not communicable. [6] Relatively few case reports implicating C. echinulata are available. Of those that are, one prototypical case from 2005 reported a fatal rhinocerebral infection in a 15-year-old boy suffering from acute leukaemia. [6] Biopsy of the infected nasal tissue showed signs of necrosis and vascular invasion. [6]
Cunninghamella echinulata, like other members of the genus, exhibit strong resistance to the antifungal polyene, amphotericin B with a MIC (Minimum Inhibitory Concentration) ranging from 4-16μg/mL that varies according to strain. [6] Strains of C. echinulata also display greater tolerance to itraconazole and posaconazole than other members of the Mucorales. [6] The antifungal agent terbinafine, typically restricted to the treatment of nail and skin infections, shows a relatively low MIC ranging from 0.06 to 0.125 μg/mL. [6]
Biotechnology
It is commonly cultivated for its ability to produce GLA, [4] preferentially synthesizing R-PHM and S-HMM. [14] The fungus is able to synthesize γ-linolenic acid. [4] It also possesses the ability to bioabsorb metals, with the highest levels of bioabsorption reported 5 to 15 minutes after contact with the metals. [5] Adding NaOH to this fungus before it absorbs metals enhances the uptake of Pb, Cu and Zn. [5] These uptake rates also seem to be influenced by pH where at a pH of 7.1, Zn was the most highly absorbed metal, [5] at a pH of 4, Pb was the most highly absorbed metal [5] and at a pH of 5, Cu was the most highly absorbed metal. [5] Cunninghamella echinulata has been used to transform cortexolone to hydrocortisone. [17] Hydroxylation of biphenyl oxide has been studied in C. echinulata. [18]
References
- ^ a b c d e Webster, John (1980). Introduction to Fungi. Cambridge: Cambridge university press. pp. 230. ISBN 0-521-22888-3.
- ^
a
b
c
d
e
f
g
h
i
j
k
l
m
n Domsch, Gams, Heidi-Anderson, K.H, W, Traute (1980). Compendium of Soil Fungi (Vol I). Academic Press. p. 238.
{{ cite book}}: CS1 maint: multiple names: authors list ( link) - ^ a b c Skinner, Charles (1947). Molds, Yeasts and Actinomycetes. New York: John Wiley & Sons. p. 92.
- ^ a b c d e De-Wei, Li (2016). Biology of Microfungi. Springer. p. 555. ISBN 9783319291376.
- ^ a b c d e f g El-Morsy, El-Sayed M. (2016-12-01). "Cunninghamella echinulata a new biosorbent of metal ions from polluted water in Egypt". Mycologia. 96 (6): 1183–1189. doi: 10.2307/3762133. ISSN 0027-5514. JSTOR 3762133. PMID 21148940.
- ^ a b c d e f g h i j k LeBlanc, Robert E.; Meriden, Zina; Sutton, Deanna A.; Thompson, Elizabeth H.; Neofytos, Dionissios; Zhang, Sean X. (2013-08-01). "Cunninghamella echinulata causing fatally invasive fungal sinusitis". Diagnostic Microbiology and Infectious Disease. 76 (4): 506–509. doi: 10.1016/j.diagmicrobio.2013.03.009. ISSN 1879-0070. PMID 23602784.
- ^ a b c Mycology Guidebook. Seattle and London: University of Washington press. 1974. pp. 94, 96, 97. ISBN 0-295-95313-6.
- ^ Hawker, Lilian (1966). Fungi. London: Hutchinson University library. p. 76.
- ^ a b c d L. O.Donnell, Kerry (1979). Zygomycetes in Culture. Dept. of Botany, University of Georgia. p. 236.
- ^ Khan (1975). "Wall Structure and Germination of Spores in Cunninghamella echinulata". Journal of General Microbiology. 90: 115–124. doi: 10.1099/00221287-90-1-115.
- ^ Watanabe, Tsuneo (2010). Pictorial Atlas of Soil and Seed Fungi. New York: CRC press. p. 69.
- ^
a
b Gwynne-Vaughan, Barnes, H.C.I, B. (1927). Structure and Development of the Fungi. New York: The Macmillan company. pp. 115, 116.
{{ cite book}}: CS1 maint: multiple names: authors list ( link) - ^
a
b
c
d
e
f Pitt, Hocking, John, Ailsa (1999). Fungi and Food Spoilage (2nd ED). Springer. pp. 178–180.
ISBN
978-0-387-92206-5.
{{ cite book}}: CS1 maint: multiple names: authors list ( link) - ^ a b c d e f g h i j Freitag, D. G; Foster, R. T; Coutts, R. T; Pickard, M. A; Pasutto, F. M (1997). "Stereoselective metabolism of rac-mexiletine by the fungus Cunninghamella echinulata yields the major human metabolites hydroxymethylmexiletine and p-hydroxymexiletine". Drug Metab Dispos. 25 (6): 685–692. PMID 9193869.
- ^ a b c d Gema, H.; Kavadia, A.; Dimou, D.; Tsagou, V.; Komaitis, M.; Aggelis, G. (2002). "Production of γ-linolenic acid by Cunninghamella echinulata cultivated on glucose and orange peel". Applied Microbiology and Biotechnology. 58 (3): 303–307. doi: 10.1007/s00253-001-0910-7. ISSN 0175-7598. PMID 11935180. S2CID 2346913.
- ^ Dennis, R.W.G (1986). Fungi of the Hebrides. Kew: Royal Botanic Gardens. p. 231. ISBN 0-947643-02-8.
- ^ Manosroi, J.; Chisti, Y.; Manosroi, A. (2006). "Biotransformation of cortexolone to hydrocortisone by molds using a rapid color development assay". Prikladnaia Biokhimiia I Mikrobiologiia. 42 (5): 547–551. PMID 17066954.
- ^ Seigle-Murandi, F. M.; Krivobok, S. M. A.; Steiman, R. L.; Benoit-Guyod, J. L. A.; Thiault, G. A. (1991). "Biphenyl oxide hydroxylation by Cunninghamella echinulata". Journal of Agricultural and Food Chemistry. 39 (2): 428. doi: 10.1021/jf00002a041.