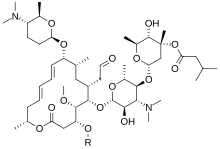
 Carrimycin mixture: R = H, acetyl, or propionyl | |
| Clinical data | |
|---|---|
| Trade names | Bite [1] |
| Other names | Isovalerylspiramycin |
| Legal status | |
| Legal status |
|
| Identifiers | |
| DrugBank | |
| UNII | |
Carrimycin is a macrolide antibiotic. It was approved by the National Medical Products Administration of China in 2019. [1] It is approved for the treatment of acute tracheal bronchitis caused by Haemophilus influenzae, Streptococcus pneumoniae, and for the treatment of acute sinusitis caused by S. pneumoniae, H. influenzae, Streptococcus pyogenes, Moraxella catarrhalis, and Staphylococcus. [2] Carrimycin is mainly used for the treatment of upper respiratory tract infections. [1]
Carrimycin is a mixture of related chemical compounds, carrimycin I through III, which are each ester derivatives of spiramycin. [3]
Carrimycin has also been investigated for the treatment of COVID-19. [2] [4]
References
- ^ a b c "Class I drug Carrimycin approved by National Medical Products Administration of China". Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences & Peking Union Medical College. July 5, 2019.
- ^
a
b Yan H, Sun J, Wang K, Wang H, Wu S, Bao L, et al. (September 2021).
"Repurposing carrimycin as an antiviral agent against human coronaviruses, including the currently pandemic SARS-CoV-2". Acta Pharmaceutica Sinica. B. 11 (9): 2850–2858.
doi:
10.1016/j.apsb.2021.02.024.
PMC
7946546.
PMID
33723501.
{{ cite journal}}: CS1 maint: overridden setting ( link) - ^ Yu LC, Dang DD, Zhuang S, Chen S, Zhuang Z, Rosenblum JS (April 2023). "Carrimycin, a first in-class anti-cancer agent, targets selenoprotein H to induce nucleolar oxidative stress and inhibit ribosome biogenesis". Cancer Pathogenesis and Therapy. 1 (2): 111–115. doi: 10.1016/j.cpt.2022.12.005. PMC 10518895. PMID 37750087.
- ^ Al-Horani RA, Kar S, Aliter KF (July 2020). "Potential Anti-COVID-19 Therapeutics that Block the Early Stage of the Viral Life Cycle: Structures, Mechanisms, and Clinical Trials". International Journal of Molecular Sciences. 21 (15): 5224. doi: 10.3390/ijms21155224. PMC 7432953. PMID 32718020.
 Carrimycin mixture: R = H, acetyl, or propionyl | |
| Clinical data | |
|---|---|
| Trade names | Bite [1] |
| Other names | Isovalerylspiramycin |
| Legal status | |
| Legal status |
|
| Identifiers | |
| DrugBank | |
| UNII | |
Carrimycin is a macrolide antibiotic. It was approved by the National Medical Products Administration of China in 2019. [1] It is approved for the treatment of acute tracheal bronchitis caused by Haemophilus influenzae, Streptococcus pneumoniae, and for the treatment of acute sinusitis caused by S. pneumoniae, H. influenzae, Streptococcus pyogenes, Moraxella catarrhalis, and Staphylococcus. [2] Carrimycin is mainly used for the treatment of upper respiratory tract infections. [1]
Carrimycin is a mixture of related chemical compounds, carrimycin I through III, which are each ester derivatives of spiramycin. [3]
Carrimycin has also been investigated for the treatment of COVID-19. [2] [4]
References
- ^ a b c "Class I drug Carrimycin approved by National Medical Products Administration of China". Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences & Peking Union Medical College. July 5, 2019.
- ^
a
b Yan H, Sun J, Wang K, Wang H, Wu S, Bao L, et al. (September 2021).
"Repurposing carrimycin as an antiviral agent against human coronaviruses, including the currently pandemic SARS-CoV-2". Acta Pharmaceutica Sinica. B. 11 (9): 2850–2858.
doi:
10.1016/j.apsb.2021.02.024.
PMC
7946546.
PMID
33723501.
{{ cite journal}}: CS1 maint: overridden setting ( link) - ^ Yu LC, Dang DD, Zhuang S, Chen S, Zhuang Z, Rosenblum JS (April 2023). "Carrimycin, a first in-class anti-cancer agent, targets selenoprotein H to induce nucleolar oxidative stress and inhibit ribosome biogenesis". Cancer Pathogenesis and Therapy. 1 (2): 111–115. doi: 10.1016/j.cpt.2022.12.005. PMC 10518895. PMID 37750087.
- ^ Al-Horani RA, Kar S, Aliter KF (July 2020). "Potential Anti-COVID-19 Therapeutics that Block the Early Stage of the Viral Life Cycle: Structures, Mechanisms, and Clinical Trials". International Journal of Molecular Sciences. 21 (15): 5224. doi: 10.3390/ijms21155224. PMC 7432953. PMID 32718020.