| Bromodomain | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
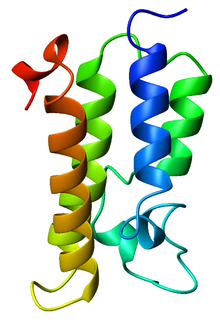
Ribbon diagram of the GCN5 bromodomain from
Saccharomyces cerevisiae, colored from blue (
N-terminus) to red (
C-terminus).
[1] | |||||||||||
| Identifiers | |||||||||||
| Symbol | Bromodomain | ||||||||||
| Pfam | PF00439 | ||||||||||
| InterPro | IPR001487 | ||||||||||
| SMART | SM00297 | ||||||||||
| PROSITE | PDOC00550 | ||||||||||
| SCOP2 | 1b91 / SCOPe / SUPFAM | ||||||||||
| CDD | cd04369 | ||||||||||
| |||||||||||
A bromodomain is an approximately 110 amino acid protein domain that recognizes acetylated lysine residues, such as those on the N-terminal tails of histones. Bromodomains, as the "readers" of lysine acetylation, are responsible in transducing the signal carried by acetylated lysine residues and translating it into various normal or abnormal phenotypes. [2] Their affinity is higher for regions where multiple acetylation sites exist in proximity. This recognition is often a prerequisite for protein-histone association and chromatin remodeling. The domain itself adopts an all-α protein fold, a bundle of four alpha helices each separated by loop regions of variable lengths that form a hydrophobic pocket that recognizes the acetyl lysine. [1] [3]
Discovery
The bromodomain was identified as a novel structural motif by John W. Tamkun and colleagues studying the Drosophila gene Brahma/brm, and showed sequence similarity to genes involved in transcriptional activation. [4] The name "bromodomain" is derived from the relationship of this domain with Brahma and is unrelated to the chemical element bromine.
Bromodomain-containing proteins
Bromodomain-containing proteins can have a wide variety of functions, ranging from histone acetyltransferase activity and chromatin remodeling to transcriptional mediation and co-activation. Of the 43 known in 2015, 11 had two bromodomains, and one protein had 6 bromodomains. [2] Preparation, biochemical analysis, and structure determination of the bromodomain containing proteins have been described in detail. [5]
Bromo- and Extra-Terminal domain (BET) family
A well-known example of a bromodomain family is the BET (Bromodomain and extraterminal domain) family. Members of this family include BRD2, BRD3, BRD4 and BRDT. [6]
Other
However proteins such as ASH1L also contain a bromodomain. Dysfunction of BRD proteins has been linked to diseases such as human squamous cell carcinoma and other forms of cancer. [7] Histone acetyltransferases, including EP300 and PCAF, have bromodomains in addition to acetyl-transferase domains. [8] [9] [10]
Not considered part of the BET family (yet containing a bromodomain) are BRD7, and BRD9.
Role in human disease
The role of bromodomains in translating a deregulated cell acetylome into disease phenotypes was recently unveiled by the development of small molecule bromodomain inhibitors. This breakthrough discovery highlighted bromodomain-containing proteins as key players in cancer biology, as well as inflammation [11] and remyelination in multiple sclerosis. [2]
Members of the BET family have been implicated as targets in both human cancer [12] [13] and multiple sclerosis. [14] BET inhibitors have shown therapeutic effects in multiple preclinical models of cancer and are currently in clinical trials in the United States. [15] Their application in multiple sclerosis is still in the preclinical stage.
Small molecule inhibitors of non-BET bromodomain proteins BRD7 and BRD9 have also been developed. [16] [17]
See also
References
- ^ a b PDB: 1e6i; Owen DJ, Ornaghi P, Yang JC, Lowe N, Evans PR, Ballario P, Neuhaus D, Filetici P, Travers AA (November 2000). "The structural basis for the recognition of acetylated histone H4 by the bromodomain of histone acetyltransferase gcn5p". EMBO J. 19 (22): 6141–9. doi: 10.1093/emboj/19.22.6141. PMC 305837. PMID 11080160.
- ^ a b c Ntranos, Achilles; Casaccia, Patrizia (2016). "Bromodomains: Translating the words of lysine acetylation into myelin injury and repair". Neuroscience Letters. 625: 4–10. doi: 10.1016/j.neulet.2015.10.015. PMC 4841751. PMID 26472704.
- ^ Zeng L, Zhou MM (February 2002). "Bromodomain: an acetyl-lysine binding domain". FEBS Lett. 513 (1): 124–8. doi: 10.1016/S0014-5793(01)03309-9. PMID 11911891. S2CID 29706103.
- ^ Tamkun JW, Deuring R, Scott MP, Kissinger M, Pattatucci AM, Kaufman TC, Kennison JA (February 1992). "brahma: a regulator of Drosophila homeotic genes structurally related to the yeast transcriptional activator SNF2/SWI2". Cell. 68 (3): 561–72. doi: 10.1016/0092-8674(92)90191-E. PMID 1346755. S2CID 27726226.
- ^ Ren, C; Zeng, L; Zhou, MM (2016). Preparation, Biochemical Analysis, and Structure Determination of the Bromodomain, an Acetyl-Lysine Binding Domain. Methods in Enzymology. Vol. 573. pp. 321–43. doi: 10.1016/bs.mie.2016.01.018. ISBN 9780128053652. PMID 27372760.
- ^ Taniguchi, Yasushi (2016-11-07). "The Bromodomain and Extra-Terminal Domain (BET) Family: Functional Anatomy of BET Paralogous Proteins". International Journal of Molecular Sciences. 17 (11): 1849. doi: 10.3390/ijms17111849. ISSN 1422-0067. PMC 5133849. PMID 27827996.
- ^ Filippakopoulos, Panagis (2012). "Histone Recognition and Large-Scale Structural Analysis of the Human Bromodomain Family". Cell. 149 (1): 214–231. doi: 10.1016/j.cell.2012.02.013. PMC 3326523. PMID 22464331.
- ^ Dhalluin, C; Carlson, J. E.; Zeng, L; He, C; Aggarwal, A. K.; Zhou, M. M.; Zhou, Ming-Ming (1999). "Structure and ligand of a histone acetyltransferase bromodomain". Nature. 399 (6735): 491–6. doi: 10.1038/20974. PMID 10365964. S2CID 1210925.
- ^ Santillan, D. A.; Theisler, C. M.; Ryan, A. S.; Popovic, R; Stuart, T; Zhou, M. M.; Alkan, S; Zeleznik-Le, N. J. (2006). "Bromodomain and histone acetyltransferase domain specificities control mixed lineage leukemia phenotype". Cancer Research. 66 (20): 10032–9. doi: 10.1158/0008-5472.CAN-06-2597. PMID 17047066.
- ^ Hay, D. A.; Fedorov, O; Martin, S; Singleton, D. C.; Tallant, C; Wells, C; Picaud, S; Philpott, M; Monteiro, O. P.; Rogers, C. M.; Conway, S. J.; Rooney, T. P.; Tumber, A; Yapp, C; Filippakopoulos, P; Bunnage, M. E.; Müller, S; Knapp, S; Schofield, C. J.; Brennan, P. E. (2014). "Discovery and optimization of small-molecule ligands for the CBP/p300 bromodomains". Journal of the American Chemical Society. 136 (26): 9308–19. doi: 10.1021/ja412434f. PMC 4183655. PMID 24946055.
- ^ Wang, Nian; Wu, Runliu; Tang, Daolin; Kang, Rui (2021-01-19). "The BET family in immunity and disease". Signal Transduction and Targeted Therapy. 6 (1): 23. doi: 10.1038/s41392-020-00384-4. ISSN 2059-3635. PMC 7813845. PMID 33462181.
- ^ Jung, Marie; Gelato, Kathy A; Fernández-Montalván, Amaury; Siegel, Stephan; Haendler, Bernard (2015-06-16). "Targeting BET bromodomains for cancer treatment". Epigenomics. 7 (3): 487–501. doi: 10.2217/epi.14.91. PMID 26077433.
- ^ Da Costa, D.; Agathanggelou, A.; Perry, T.; Weston, V.; Petermann, E.; Zlatanou, A.; Oldreive, C.; Wei, W.; Stewart, G. (2013-07-19). "BET inhibition as a single or combined therapeutic approach in primary paediatric B-precursor acute lymphoblastic leukaemia". Blood Cancer Journal. 3 (7): e126. doi: 10.1038/bcj.2013.24. PMC 3730202. PMID 23872705.
- ^ Gacias, Mar; Gerona-Navarro, Guillermo; Plotnikov, Alexander N.; Zhang, Guangtao; Zeng, Lei; Kaur, Jasbir; Moy, Gregory; Rusinova, Elena; Rodriguez, Yoel (2014). "Selective Chemical Modulation of Gene Transcription Favors Oligodendrocyte Lineage Progression". Chemistry & Biology. 21 (7): 841–854. doi: 10.1016/j.chembiol.2014.05.009. ISSN 1074-5521. PMC 4104156. PMID 24954007.
- ^ Shi, Junwei (2014). "The Mechanisms behind the Therapeutic Activity of BET Bromodomain Inhibition". Molecular Cell. 54 (5): 728–736. doi: 10.1016/j.molcel.2014.05.016. PMC 4236231. PMID 24905006.
- ^ Clark, P. G.; Vieira, L. C.; Tallant, C; Fedorov, O; Singleton, D. C.; Rogers, C. M.; Monteiro, O. P.; Bennett, J. M.; Baronio, R; Müller, S; Daniels, D. L.; Méndez, J; Knapp, S; Brennan, P. E.; Dixon, D. J. (2015). "LP99: Discovery and Synthesis of the First Selective BRD7/9 Bromodomain Inhibitor". Angewandte Chemie International Edition. 54 (21): 6217–21. doi: 10.1002/anie.201501394. PMC 4449114. PMID 25864491.
- ^ Theodoulou, N. H.; Bamborough, P; Bannister, A. J.; Becher, I; Bit, R. A.; Che, K. H.; Chung, C. W.; Dittmann, A; Drewes, G; Drewry, D. H.; Gordon, L; Grandi, P; Leveridge, M; Lindon, M; Michon, A. M.; Molnar, J; Robson, S. C.; Tomkinson, N. C.; Kouzarides, T; Prinjha, R. K.; Humphreys, P. G. (2015). "The Discovery of I-BRD9, a Selective Cell Active Chemical Probe for Bromodomain Containing Protein 9 Inhibition". Journal of Medicinal Chemistry. 59 (4): 1425–39. doi: 10.1021/acs.jmedchem.5b00256. PMC 7354103. PMID 25856009.
| Bromodomain | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|

Ribbon diagram of the GCN5 bromodomain from
Saccharomyces cerevisiae, colored from blue (
N-terminus) to red (
C-terminus).
[1] | |||||||||||
| Identifiers | |||||||||||
| Symbol | Bromodomain | ||||||||||
| Pfam | PF00439 | ||||||||||
| InterPro | IPR001487 | ||||||||||
| SMART | SM00297 | ||||||||||
| PROSITE | PDOC00550 | ||||||||||
| SCOP2 | 1b91 / SCOPe / SUPFAM | ||||||||||
| CDD | cd04369 | ||||||||||
| |||||||||||
A bromodomain is an approximately 110 amino acid protein domain that recognizes acetylated lysine residues, such as those on the N-terminal tails of histones. Bromodomains, as the "readers" of lysine acetylation, are responsible in transducing the signal carried by acetylated lysine residues and translating it into various normal or abnormal phenotypes. [2] Their affinity is higher for regions where multiple acetylation sites exist in proximity. This recognition is often a prerequisite for protein-histone association and chromatin remodeling. The domain itself adopts an all-α protein fold, a bundle of four alpha helices each separated by loop regions of variable lengths that form a hydrophobic pocket that recognizes the acetyl lysine. [1] [3]
Discovery
The bromodomain was identified as a novel structural motif by John W. Tamkun and colleagues studying the Drosophila gene Brahma/brm, and showed sequence similarity to genes involved in transcriptional activation. [4] The name "bromodomain" is derived from the relationship of this domain with Brahma and is unrelated to the chemical element bromine.
Bromodomain-containing proteins
Bromodomain-containing proteins can have a wide variety of functions, ranging from histone acetyltransferase activity and chromatin remodeling to transcriptional mediation and co-activation. Of the 43 known in 2015, 11 had two bromodomains, and one protein had 6 bromodomains. [2] Preparation, biochemical analysis, and structure determination of the bromodomain containing proteins have been described in detail. [5]
Bromo- and Extra-Terminal domain (BET) family
A well-known example of a bromodomain family is the BET (Bromodomain and extraterminal domain) family. Members of this family include BRD2, BRD3, BRD4 and BRDT. [6]
Other
However proteins such as ASH1L also contain a bromodomain. Dysfunction of BRD proteins has been linked to diseases such as human squamous cell carcinoma and other forms of cancer. [7] Histone acetyltransferases, including EP300 and PCAF, have bromodomains in addition to acetyl-transferase domains. [8] [9] [10]
Not considered part of the BET family (yet containing a bromodomain) are BRD7, and BRD9.
Role in human disease
The role of bromodomains in translating a deregulated cell acetylome into disease phenotypes was recently unveiled by the development of small molecule bromodomain inhibitors. This breakthrough discovery highlighted bromodomain-containing proteins as key players in cancer biology, as well as inflammation [11] and remyelination in multiple sclerosis. [2]
Members of the BET family have been implicated as targets in both human cancer [12] [13] and multiple sclerosis. [14] BET inhibitors have shown therapeutic effects in multiple preclinical models of cancer and are currently in clinical trials in the United States. [15] Their application in multiple sclerosis is still in the preclinical stage.
Small molecule inhibitors of non-BET bromodomain proteins BRD7 and BRD9 have also been developed. [16] [17]
See also
References
- ^ a b PDB: 1e6i; Owen DJ, Ornaghi P, Yang JC, Lowe N, Evans PR, Ballario P, Neuhaus D, Filetici P, Travers AA (November 2000). "The structural basis for the recognition of acetylated histone H4 by the bromodomain of histone acetyltransferase gcn5p". EMBO J. 19 (22): 6141–9. doi: 10.1093/emboj/19.22.6141. PMC 305837. PMID 11080160.
- ^ a b c Ntranos, Achilles; Casaccia, Patrizia (2016). "Bromodomains: Translating the words of lysine acetylation into myelin injury and repair". Neuroscience Letters. 625: 4–10. doi: 10.1016/j.neulet.2015.10.015. PMC 4841751. PMID 26472704.
- ^ Zeng L, Zhou MM (February 2002). "Bromodomain: an acetyl-lysine binding domain". FEBS Lett. 513 (1): 124–8. doi: 10.1016/S0014-5793(01)03309-9. PMID 11911891. S2CID 29706103.
- ^ Tamkun JW, Deuring R, Scott MP, Kissinger M, Pattatucci AM, Kaufman TC, Kennison JA (February 1992). "brahma: a regulator of Drosophila homeotic genes structurally related to the yeast transcriptional activator SNF2/SWI2". Cell. 68 (3): 561–72. doi: 10.1016/0092-8674(92)90191-E. PMID 1346755. S2CID 27726226.
- ^ Ren, C; Zeng, L; Zhou, MM (2016). Preparation, Biochemical Analysis, and Structure Determination of the Bromodomain, an Acetyl-Lysine Binding Domain. Methods in Enzymology. Vol. 573. pp. 321–43. doi: 10.1016/bs.mie.2016.01.018. ISBN 9780128053652. PMID 27372760.
- ^ Taniguchi, Yasushi (2016-11-07). "The Bromodomain and Extra-Terminal Domain (BET) Family: Functional Anatomy of BET Paralogous Proteins". International Journal of Molecular Sciences. 17 (11): 1849. doi: 10.3390/ijms17111849. ISSN 1422-0067. PMC 5133849. PMID 27827996.
- ^ Filippakopoulos, Panagis (2012). "Histone Recognition and Large-Scale Structural Analysis of the Human Bromodomain Family". Cell. 149 (1): 214–231. doi: 10.1016/j.cell.2012.02.013. PMC 3326523. PMID 22464331.
- ^ Dhalluin, C; Carlson, J. E.; Zeng, L; He, C; Aggarwal, A. K.; Zhou, M. M.; Zhou, Ming-Ming (1999). "Structure and ligand of a histone acetyltransferase bromodomain". Nature. 399 (6735): 491–6. doi: 10.1038/20974. PMID 10365964. S2CID 1210925.
- ^ Santillan, D. A.; Theisler, C. M.; Ryan, A. S.; Popovic, R; Stuart, T; Zhou, M. M.; Alkan, S; Zeleznik-Le, N. J. (2006). "Bromodomain and histone acetyltransferase domain specificities control mixed lineage leukemia phenotype". Cancer Research. 66 (20): 10032–9. doi: 10.1158/0008-5472.CAN-06-2597. PMID 17047066.
- ^ Hay, D. A.; Fedorov, O; Martin, S; Singleton, D. C.; Tallant, C; Wells, C; Picaud, S; Philpott, M; Monteiro, O. P.; Rogers, C. M.; Conway, S. J.; Rooney, T. P.; Tumber, A; Yapp, C; Filippakopoulos, P; Bunnage, M. E.; Müller, S; Knapp, S; Schofield, C. J.; Brennan, P. E. (2014). "Discovery and optimization of small-molecule ligands for the CBP/p300 bromodomains". Journal of the American Chemical Society. 136 (26): 9308–19. doi: 10.1021/ja412434f. PMC 4183655. PMID 24946055.
- ^ Wang, Nian; Wu, Runliu; Tang, Daolin; Kang, Rui (2021-01-19). "The BET family in immunity and disease". Signal Transduction and Targeted Therapy. 6 (1): 23. doi: 10.1038/s41392-020-00384-4. ISSN 2059-3635. PMC 7813845. PMID 33462181.
- ^ Jung, Marie; Gelato, Kathy A; Fernández-Montalván, Amaury; Siegel, Stephan; Haendler, Bernard (2015-06-16). "Targeting BET bromodomains for cancer treatment". Epigenomics. 7 (3): 487–501. doi: 10.2217/epi.14.91. PMID 26077433.
- ^ Da Costa, D.; Agathanggelou, A.; Perry, T.; Weston, V.; Petermann, E.; Zlatanou, A.; Oldreive, C.; Wei, W.; Stewart, G. (2013-07-19). "BET inhibition as a single or combined therapeutic approach in primary paediatric B-precursor acute lymphoblastic leukaemia". Blood Cancer Journal. 3 (7): e126. doi: 10.1038/bcj.2013.24. PMC 3730202. PMID 23872705.
- ^ Gacias, Mar; Gerona-Navarro, Guillermo; Plotnikov, Alexander N.; Zhang, Guangtao; Zeng, Lei; Kaur, Jasbir; Moy, Gregory; Rusinova, Elena; Rodriguez, Yoel (2014). "Selective Chemical Modulation of Gene Transcription Favors Oligodendrocyte Lineage Progression". Chemistry & Biology. 21 (7): 841–854. doi: 10.1016/j.chembiol.2014.05.009. ISSN 1074-5521. PMC 4104156. PMID 24954007.
- ^ Shi, Junwei (2014). "The Mechanisms behind the Therapeutic Activity of BET Bromodomain Inhibition". Molecular Cell. 54 (5): 728–736. doi: 10.1016/j.molcel.2014.05.016. PMC 4236231. PMID 24905006.
- ^ Clark, P. G.; Vieira, L. C.; Tallant, C; Fedorov, O; Singleton, D. C.; Rogers, C. M.; Monteiro, O. P.; Bennett, J. M.; Baronio, R; Müller, S; Daniels, D. L.; Méndez, J; Knapp, S; Brennan, P. E.; Dixon, D. J. (2015). "LP99: Discovery and Synthesis of the First Selective BRD7/9 Bromodomain Inhibitor". Angewandte Chemie International Edition. 54 (21): 6217–21. doi: 10.1002/anie.201501394. PMC 4449114. PMID 25864491.
- ^ Theodoulou, N. H.; Bamborough, P; Bannister, A. J.; Becher, I; Bit, R. A.; Che, K. H.; Chung, C. W.; Dittmann, A; Drewes, G; Drewry, D. H.; Gordon, L; Grandi, P; Leveridge, M; Lindon, M; Michon, A. M.; Molnar, J; Robson, S. C.; Tomkinson, N. C.; Kouzarides, T; Prinjha, R. K.; Humphreys, P. G. (2015). "The Discovery of I-BRD9, a Selective Cell Active Chemical Probe for Bromodomain Containing Protein 9 Inhibition". Journal of Medicinal Chemistry. 59 (4): 1425–39. doi: 10.1021/acs.jmedchem.5b00256. PMC 7354103. PMID 25856009.