| |

| |
| Names | |
|---|---|
|
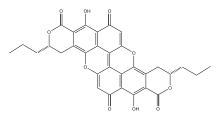
Preferred IUPAC name
(3S,11S)-8,16-Dihydroxy-3,11-dipropyl-3,4,11,12-tetrahydro-1H,7H-pyrano[4,3-h]pyrano[4′,3′:5,6]xantheno[2,1,9,8-klmna]xanthene-1,7,9,15-tetrone | |
| Other names
Xylindene
(3S,11S)-3,4,11,12-Tetrahydro-8,16-dihydroxy-3,11-dipropyl-1H,7H-dipyrano[4,3-a:4',3'-j]-peri-xanthenoxanthene-1,7,9,15-tetrone peri-xanthenoxanthene-2,8-dicarboxy-lic acid 4,10-dihydro-3,9-dihydroxy-1,7-bis(2 S-hydroxy-pentyl)-4,10-dioxo-di δ-lactone | |
| Identifiers | |
3D model (
JSmol)
|
|
| ChemSpider | |
PubChem
CID
|
|
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C32H24O10 | |
| Molar mass | 568.534 g·mol−1 |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Xylindein is a quinone pigment, a dimeric naphthoquinone derivative. It is produced by fungi in the genus Chlorociboria. This pigment causes green staining of wood infected by the fungi.
This pigment was firstly extracted in 1868 by Paul Thénard from wood and resembled indigo, so he called it xylindéine. Combination of xyl- (wood) and indé ( indigo) + -ine. [1] [2]
- ^ Xylindein at Merriam-Webster dictionary
- ^ Thenard, Paul; Alphonse Rommier (1868). "Sur un nouvelle matière colorante appelée xylindeine et extraite de certains bois morts". Comptes rendus hebdomadaires des séances de l'Académie des Sciences (in French). 66. Paris: 108–109. ISSN 0001-4036.
- Saikawa, Yoko; Watanabe, Takashi; Hashimoto, Kimiko; Nakata, Masaya (October 2000). "Absolute configuration and tautomeric structure of xylindein, a blue-green pigment of Chlorociboria species". Phytochemistry. 55 (3). Elsevier: 237–240. Bibcode: 2000PChem..55..237S. doi: 10.1016/S0031-9422(00)00282-X. PMID 11142849.
- Donner, Christopher; Cuzzupe, Anthony; Falzon, Cheryl; Gill, Melvyn (April 2012). "Investigations towards the synthesis of xylindein, a blue-green pigment from the fungus Chlorociboria aeruginosa". Tetrahedron. 68 (13). Elsevier: 2799–2805. doi: 10.1016/j.tet.2012.02.009.
- Robinson, Sara; Tudor, Daniela; Snider, Hilary; Cooper, Paul (March 2012). "Stimulating growth and xylindein production of Chlorociboria aeruginascens in agar-based systems". AMB Express. 2. Springer: 15. doi: 10.1186/2191-0855-2-15. PMC 3350399. PMID 22409931.
- Ostroverkhova, Oksana; Robinson, Sara; Gutierrez, Sarath Vega; Schenck, Jonathan Van; Giesbers, Gregory (2018). "Fungi-Derived Pigments for Sustainable Organic (Opto)Electronics". MRS Advances. 3 (59): 3459–3464. doi: 10.1557/adv.2018.446. ISSN 2059-8521. S2CID 53646580.
-
 Media related to
Xylindein at Wikimedia Commons
Media related to
Xylindein at Wikimedia Commons

| |

| |
| Names | |
|---|---|
|
Preferred IUPAC name
(3S,11S)-8,16-Dihydroxy-3,11-dipropyl-3,4,11,12-tetrahydro-1H,7H-pyrano[4,3-h]pyrano[4′,3′:5,6]xantheno[2,1,9,8-klmna]xanthene-1,7,9,15-tetrone | |
| Other names
Xylindene
(3S,11S)-3,4,11,12-Tetrahydro-8,16-dihydroxy-3,11-dipropyl-1H,7H-dipyrano[4,3-a:4',3'-j]-peri-xanthenoxanthene-1,7,9,15-tetrone peri-xanthenoxanthene-2,8-dicarboxy-lic acid 4,10-dihydro-3,9-dihydroxy-1,7-bis(2 S-hydroxy-pentyl)-4,10-dioxo-di δ-lactone | |
| Identifiers | |
3D model (
JSmol)
|
|
| ChemSpider | |
PubChem
CID
|
|
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C32H24O10 | |
| Molar mass | 568.534 g·mol−1 |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Xylindein is a quinone pigment, a dimeric naphthoquinone derivative. It is produced by fungi in the genus Chlorociboria. This pigment causes green staining of wood infected by the fungi.
This pigment was firstly extracted in 1868 by Paul Thénard from wood and resembled indigo, so he called it xylindéine. Combination of xyl- (wood) and indé ( indigo) + -ine. [1] [2]
- ^ Xylindein at Merriam-Webster dictionary
- ^ Thenard, Paul; Alphonse Rommier (1868). "Sur un nouvelle matière colorante appelée xylindeine et extraite de certains bois morts". Comptes rendus hebdomadaires des séances de l'Académie des Sciences (in French). 66. Paris: 108–109. ISSN 0001-4036.
- Saikawa, Yoko; Watanabe, Takashi; Hashimoto, Kimiko; Nakata, Masaya (October 2000). "Absolute configuration and tautomeric structure of xylindein, a blue-green pigment of Chlorociboria species". Phytochemistry. 55 (3). Elsevier: 237–240. Bibcode: 2000PChem..55..237S. doi: 10.1016/S0031-9422(00)00282-X. PMID 11142849.
- Donner, Christopher; Cuzzupe, Anthony; Falzon, Cheryl; Gill, Melvyn (April 2012). "Investigations towards the synthesis of xylindein, a blue-green pigment from the fungus Chlorociboria aeruginosa". Tetrahedron. 68 (13). Elsevier: 2799–2805. doi: 10.1016/j.tet.2012.02.009.
- Robinson, Sara; Tudor, Daniela; Snider, Hilary; Cooper, Paul (March 2012). "Stimulating growth and xylindein production of Chlorociboria aeruginascens in agar-based systems". AMB Express. 2. Springer: 15. doi: 10.1186/2191-0855-2-15. PMC 3350399. PMID 22409931.
- Ostroverkhova, Oksana; Robinson, Sara; Gutierrez, Sarath Vega; Schenck, Jonathan Van; Giesbers, Gregory (2018). "Fungi-Derived Pigments for Sustainable Organic (Opto)Electronics". MRS Advances. 3 (59): 3459–3464. doi: 10.1557/adv.2018.446. ISSN 2059-8521. S2CID 53646580.
-
 Media related to
Xylindein at Wikimedia Commons
Media related to
Xylindein at Wikimedia Commons