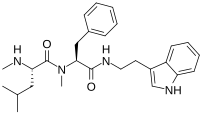
The xenortides (A-D) are a class of linear peptides isolated from the bacterium Xenorhabdus nematophila, [1] [2] a symbiont of the entomopathogenic nematode Steinernema carpocapsae. This class of compounds is known for their insect virulence and cytotoxic biological activities. The tryptamide containing compounds (xenortides B and D) show higher biological activity than the phenylethylamides (xenortides A and C). The most biologically active compound was found to be xenortide B with a potency of less than 1.6 μM activity against Trypanosoma brucei rhodesiense ( sleeping sickness) and Plasmodium falciparum ( malaria), however it is also the most toxic to mammalian cells which limits its viability as a treatment. [1]
The biosynthesis of xenortides A-D consists of two non-ribosomal peptide synthases (NRPS) coded by genes XndA and XndB, as well as upstream NADH flavin reductase, and a D- aminopeptidase. The first NRPS (XndA) consists of a condensation, adenylation, methylation, and thiolation domain, and has been implicated for the loading of N-methylleucine (xenortides A-B) or N-methylvaline (xenortides C-D). The second NRPS (XndB) consists of a condensation, adenylation, methylation, thiolation, and terminal condensation domains. XndB has been implicated in elongation with N-methylphenylalanine, as well as the final condensation of the enzyme-bound peptide with either decarboxylated phenylalanine (xenortides A and C) or decarboxylated tryptophan (xenortides B and D), ending the biosynthesis. [1]

- ^ a b c d Reimer, Daniela; Nollmann, Friederike I.; Schultz, Katharina; Kaiser, Marcel; Bode, Helge B. (2014-08-22). "Xenortide Biosynthesis by Entomopathogenic Xenorhabdus nematophila". Journal of Natural Products. 77 (8): 1976–1980. doi: 10.1021/np500390b. ISSN 0163-3864. PMID 25080196.
- ^ Lang, Gerhard; Kalvelage, Tim; Peters, Arne; Wiese, Jutta; Imhoff, Johannes F. (2008-06-01). "Linear and Cyclic Peptides from the Entomopathogenic Bacterium Xenorhabdus nematophilus". Journal of Natural Products. 71 (6): 1074–1077. doi: 10.1021/np800053n. ISSN 0163-3864. PMID 18491867.
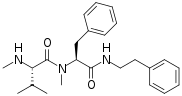
The xenortides (A-D) are a class of linear peptides isolated from the bacterium Xenorhabdus nematophila, [1] [2] a symbiont of the entomopathogenic nematode Steinernema carpocapsae. This class of compounds is known for their insect virulence and cytotoxic biological activities. The tryptamide containing compounds (xenortides B and D) show higher biological activity than the phenylethylamides (xenortides A and C). The most biologically active compound was found to be xenortide B with a potency of less than 1.6 μM activity against Trypanosoma brucei rhodesiense ( sleeping sickness) and Plasmodium falciparum ( malaria), however it is also the most toxic to mammalian cells which limits its viability as a treatment. [1]
The biosynthesis of xenortides A-D consists of two non-ribosomal peptide synthases (NRPS) coded by genes XndA and XndB, as well as upstream NADH flavin reductase, and a D- aminopeptidase. The first NRPS (XndA) consists of a condensation, adenylation, methylation, and thiolation domain, and has been implicated for the loading of N-methylleucine (xenortides A-B) or N-methylvaline (xenortides C-D). The second NRPS (XndB) consists of a condensation, adenylation, methylation, thiolation, and terminal condensation domains. XndB has been implicated in elongation with N-methylphenylalanine, as well as the final condensation of the enzyme-bound peptide with either decarboxylated phenylalanine (xenortides A and C) or decarboxylated tryptophan (xenortides B and D), ending the biosynthesis. [1]

- ^ a b c d Reimer, Daniela; Nollmann, Friederike I.; Schultz, Katharina; Kaiser, Marcel; Bode, Helge B. (2014-08-22). "Xenortide Biosynthesis by Entomopathogenic Xenorhabdus nematophila". Journal of Natural Products. 77 (8): 1976–1980. doi: 10.1021/np500390b. ISSN 0163-3864. PMID 25080196.
- ^ Lang, Gerhard; Kalvelage, Tim; Peters, Arne; Wiese, Jutta; Imhoff, Johannes F. (2008-06-01). "Linear and Cyclic Peptides from the Entomopathogenic Bacterium Xenorhabdus nematophilus". Journal of Natural Products. 71 (6): 1074–1077. doi: 10.1021/np800053n. ISSN 0163-3864. PMID 18491867.