| |||
| Names | |||
|---|---|---|---|
|
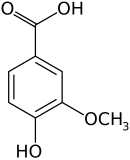
Preferred IUPAC name
4-Hydroxy-3-methoxybenzoic acid | |||
| Other names
4-Hydroxy-m-anisic acid, Vanillate
| |||
| Identifiers | |||
3D model (
JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.061 | ||
| KEGG | |||
PubChem
CID
|
|||
| UNII | |||
CompTox Dashboard (
EPA)
|
|||
| |||
| |||
| Properties | |||
| C8H8O4 | |||
| Molar mass | 168.148 g·mol−1 | ||
| Appearance | White to light yellow powder or crystals | ||
| Melting point | 210 to 213 °C (410 to 415 °F; 483 to 486 K) | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Related compounds
|
Vanillin, vanillyl alcohol | ||
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |||
Vanillic acid (4-hydroxy-3-methoxybenzoic acid) is a dihydroxybenzoic acid derivative used as a flavoring agent. It is an oxidized form of vanillin. It is also an intermediate in the production of vanillin from ferulic acid. [2] [3]
Occurrence in nature
The highest amount of vanillic acid in plants known so far is found in the root of Angelica sinensis, [4] an herb indigenous to China, which is used in traditional Chinese medicine.
Occurrences in food
Açaí oil, obtained from the fruit of the açaí palm ( Euterpe oleracea), is rich in vanillic acid (1616±94 mg/kg). [5] It is one of the main natural phenols in argan oil.[ citation needed] It is also found in wine and vinegar. [6]
Metabolism
Vanillic acid is one of the main catechins metabolites found in humans after consumption of green tea infusions. [7]
Synthesis
Vanillic acid can be obtained from the oxidation of vanillin by various oxidizing agents. With Pd/C, NaBH4, and KOH as the oxidizing agent, the conversion was reported to occur in ~89% yield. [8]
References
- ^ "Vanillic acid (4-hydroxy-3-methoxybenzoic acid)". chemicalland21.com. Retrieved 2009-01-28.
- ^ Lesage-Meessen L, Delattre M, Haon M, Thibault JF, Ceccaldi BC, Brunerie P, Asther M (October 1996). "A two-step bioconversion process for vanillin production from ferulic acid combining Aspergillus niger and Pycnoporus cinnabarinus". J. Biotechnol. 50 (2–3): 107–113. doi: 10.1016/0168-1656(96)01552-0. PMID 8987621.
- ^ Civolani C, Barghini P, Roncetti AR, Ruzzi M, Schiesser A (June 2000). "Bioconversion of ferulic acid into vanillic acid by means of a vanillate-negative mutant of Pseudomonas fluorescens strain BF13". Appl. Environ. Microbiol. 66 (6): 2311–2317. Bibcode: 2000ApEnM..66.2311C. doi: 10.1128/AEM.66.6.2311-2317.2000. PMC 110519. PMID 10831404.
- ^ Duke, JA (1992). Handbook of phytochemical constituents of GRAS herbs and other economic plants. CRC Press, 999 edition. ISBN 978-0-8493-3865-6. Archived from the original on 2015-09-23. Retrieved 2012-01-07.
- ^ Pacheco-Palencia LA, Mertens-Talcott S, Talcott ST (Jun 2008). "Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from Açaí (Euterpe oleracea Mart.)". J Agric Food Chem. 56 (12): 4631–4636. doi: 10.1021/jf800161u. PMID 18522407.
- ^ Gálvez MC, Barroso CG, Pérez-Bustamante JA (1994). "Analysis of polyphenolic compounds of different vinegar samples". Zeitschrift für Lebensmittel-Untersuchung und -Forschung. 199: 29–31. doi: 10.1007/BF01192948. S2CID 91784893.
- ^ Pietta PG, Simonetti P, Gardana C, Brusamolino A, Morazzoni P, Bombardelli E (1998). "Catechin metabolites after intake of green tea infusions". BioFactors. 8 (1–2): 111–8. doi: 10.1002/biof.5520080119. PMID 9699018. S2CID 37684286.
- ^ Lim M, Yoon CM, An G, Rhee H (2007). "Environmentally benign oxidation reaction of aldehydes to their corresponding carboxylic acids using Pd/C with NaBH4 and KOH". Tetrahedron Lett. 48 (22): 3835–3839. doi: 10.1016/j.tetlet.2007.03.151.
| |||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
4-Hydroxy-3-methoxybenzoic acid | |||
| Other names
4-Hydroxy-m-anisic acid, Vanillate
| |||
| Identifiers | |||
3D model (
JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.061 | ||
| KEGG | |||
PubChem
CID
|
|||
| UNII | |||
CompTox Dashboard (
EPA)
|
|||
| |||
| |||
| Properties | |||
| C8H8O4 | |||
| Molar mass | 168.148 g·mol−1 | ||
| Appearance | White to light yellow powder or crystals | ||
| Melting point | 210 to 213 °C (410 to 415 °F; 483 to 486 K) | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Related compounds
|
Vanillin, vanillyl alcohol | ||
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |||
Vanillic acid (4-hydroxy-3-methoxybenzoic acid) is a dihydroxybenzoic acid derivative used as a flavoring agent. It is an oxidized form of vanillin. It is also an intermediate in the production of vanillin from ferulic acid. [2] [3]
Occurrence in nature
The highest amount of vanillic acid in plants known so far is found in the root of Angelica sinensis, [4] an herb indigenous to China, which is used in traditional Chinese medicine.
Occurrences in food
Açaí oil, obtained from the fruit of the açaí palm ( Euterpe oleracea), is rich in vanillic acid (1616±94 mg/kg). [5] It is one of the main natural phenols in argan oil.[ citation needed] It is also found in wine and vinegar. [6]
Metabolism
Vanillic acid is one of the main catechins metabolites found in humans after consumption of green tea infusions. [7]
Synthesis
Vanillic acid can be obtained from the oxidation of vanillin by various oxidizing agents. With Pd/C, NaBH4, and KOH as the oxidizing agent, the conversion was reported to occur in ~89% yield. [8]
References
- ^ "Vanillic acid (4-hydroxy-3-methoxybenzoic acid)". chemicalland21.com. Retrieved 2009-01-28.
- ^ Lesage-Meessen L, Delattre M, Haon M, Thibault JF, Ceccaldi BC, Brunerie P, Asther M (October 1996). "A two-step bioconversion process for vanillin production from ferulic acid combining Aspergillus niger and Pycnoporus cinnabarinus". J. Biotechnol. 50 (2–3): 107–113. doi: 10.1016/0168-1656(96)01552-0. PMID 8987621.
- ^ Civolani C, Barghini P, Roncetti AR, Ruzzi M, Schiesser A (June 2000). "Bioconversion of ferulic acid into vanillic acid by means of a vanillate-negative mutant of Pseudomonas fluorescens strain BF13". Appl. Environ. Microbiol. 66 (6): 2311–2317. Bibcode: 2000ApEnM..66.2311C. doi: 10.1128/AEM.66.6.2311-2317.2000. PMC 110519. PMID 10831404.
- ^ Duke, JA (1992). Handbook of phytochemical constituents of GRAS herbs and other economic plants. CRC Press, 999 edition. ISBN 978-0-8493-3865-6. Archived from the original on 2015-09-23. Retrieved 2012-01-07.
- ^ Pacheco-Palencia LA, Mertens-Talcott S, Talcott ST (Jun 2008). "Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from Açaí (Euterpe oleracea Mart.)". J Agric Food Chem. 56 (12): 4631–4636. doi: 10.1021/jf800161u. PMID 18522407.
- ^ Gálvez MC, Barroso CG, Pérez-Bustamante JA (1994). "Analysis of polyphenolic compounds of different vinegar samples". Zeitschrift für Lebensmittel-Untersuchung und -Forschung. 199: 29–31. doi: 10.1007/BF01192948. S2CID 91784893.
- ^ Pietta PG, Simonetti P, Gardana C, Brusamolino A, Morazzoni P, Bombardelli E (1998). "Catechin metabolites after intake of green tea infusions". BioFactors. 8 (1–2): 111–8. doi: 10.1002/biof.5520080119. PMID 9699018. S2CID 37684286.
- ^ Lim M, Yoon CM, An G, Rhee H (2007). "Environmentally benign oxidation reaction of aldehydes to their corresponding carboxylic acids using Pd/C with NaBH4 and KOH". Tetrahedron Lett. 48 (22): 3835–3839. doi: 10.1016/j.tetlet.2007.03.151.