 | |
| Clinical data | |
|---|---|
| Trade names | Sovaldi, Soforal, others [1] |
| Other names | PSI-7977; GS-7977 |
| AHFS/ Drugs.com | Monograph |
| MedlinePlus | a614014 |
| License data |
|
|
Pregnancy category |
|
|
Routes of administration | by mouth [3] |
| Drug class | HCV polymerase inhibitor |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 92% |
| Protein binding | 61–65% |
| Metabolism | Quickly activated to triphosphate ( CatA/ CES1, HIST1, phosphorylation) |
| Elimination half-life | 0.4 hrs (sofosbuvir) 27 hrs (inactive metabolite GS-331007) |
| Excretion | 80% urine, 14% feces (mostly as GS-331007) |
| Identifiers | |
| |
| Chemical and physical data | |
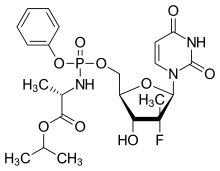
| Formula | C22H29FN3O9P |
| Molar mass | 529.458 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
Sofosbuvir, sold under the brand name Sovaldi among others, is a medication used to treat hepatitis C. [3] It is only recommended with some combination of ribavirin, peginterferon-alfa, simeprevir, ledipasvir, daclatasvir, or velpatasvir. [7] [8] Cure rates are 30 to 97% depending on the type of hepatitis C virus involved. [9] Safety during pregnancy is unclear; some of the medications used in combination may result in harm to the baby. [9] It is taken by mouth. [3]
Common side effects include feeling tired, headache, nausea, and trouble sleeping. [3] Side effects are generally more common in interferon-containing regimens. [10]: 7 Sofosbuvir may reactivate hepatitis B in those who have been previously infected. [11] In combination with ledipasvir, daclatasvir or simeprevir it is not recommended with amiodarone due to the risk of an abnormally slow heartbeat. [10] Sofosbuvir is in the nucleotide analog family of medication and works by blocking the hepatitis C NS5B protein. [12]
Sofosbuvir was discovered in 2007, and approved for medical use in the United States in 2013. [10] [7] [13] It is on the World Health Organization's List of Essential Medicines. [14] As of 2016 [update], a 12-week course of treatment costs about US$84,000 in the United States, US$53,000 in the United Kingdom, US$45,000 in Canada, and about US$500 in India. [15] Over 60,000 people were treated with sofosbuvir in its first 30 weeks being sold in the United States. [16] The patent is not recognized by Egypt, were generic versions are made. [17]
- ^ Divya Rajagopal for the Economic Times. Sept 12, 2015. Can Indian generic makers find gold with a blockbuster Hepatitis C drug? Archived 20 March 2016 at the Wayback Machine
- ^ a b "Sofosbuvir (Sovaldi) Use During Pregnancy". Drugs.com. 16 December 2019. Archived from the original on 5 February 2020. Retrieved 5 February 2020.
- ^ a b c d e "Sofosbuvir". The American Society of Health-System Pharmacists. Archived from the original on 2016-12-01. Retrieved November 30, 2016.
- ^ "Sovaldi 400 mg film coated tablets - Summary of Product Characteristics (SmPC)". (emc). 6 November 2019. Archived from the original on 22 October 2020. Retrieved 18 April 2020.
- ^ a b "SOFOSBUVIR = SOF oral - Essential drugs". medicalguidelines.msf.org. Archived from the original on 26 November 2020. Retrieved 25 August 2020.
- ^ "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 29 July 2020. Retrieved 15 September 2020.
- ^ a b "Sofosbuvir (Sovaldi) - Treatment - Hepatitis C Online". www.hepatitisc.uw.edu. Archived from the original on 23 December 2016. Retrieved 8 January 2017.
-
^ FDA, News Release (June 28, 2016).
"FDA approves Epclusa for treatment of chronic Hepatitis C virus infection".
Archived from the original on June 3, 2017.
{{ cite journal}}: Cite journal requires|journal=( help) - ^ a b "Sovaldi 400 mg film coated tablets - Summary of Product Characteristics (SPC) - (eMC)". www.medicines.org.uk. Archived from the original on 31 January 2017. Retrieved 8 January 2017.
- ^ a b c "Sovaldi- sofosbuvir tablet, film coated Sovaldi- sofosbuvir pellet". DailyMed. 27 September 2019. Archived from the original on 21 September 2020. Retrieved 4 February 2020.
- ^ "Direct-Acting Antivirals for Hepatitis C: Drug Safety Communication - Risk of Hepatitis B Reactivating". FDA. 4 October 2016. Archived from the original on 6 October 2016. Retrieved 6 October 2016.
- ^ "Sovaldi 400 mg film coated tablets - Summary of Product Characteristics". UK Electronic Medicines Compendium. September 2016. Archived from the original on 10 November 2016. Retrieved 10 November 2016.
- ^ Gounder, Celine (9 December 2013). "A Better Treatment for Hepatitis C". The New Yorker. Archived from the original on 20 September 2016.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl: 10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Hill A, Simmons B, Gotham D, Fortunak J (January 2016). "Rapid reductions in prices for generic sofosbuvir and daclatasvir to treat hepatitis C". Journal of Virus Eradication. 2 (1): 28–31. PMC 4946692. PMID 27482432.
- ^ Alonso-Zaldivar, Ricardo (July 29, 2014). "Gilead's Sovaldi prescribed more than all other hepatitis C drugs combined". Mercury News. Archived from the original on May 23, 2015.
- ^ Radu, Alexandra (28 Jul 2021). "Affordable hepatitis drug offers new hope to millions". www.aljazeera.com. Archived from the original on 1 June 2023. Retrieved 10 September 2023.
 | |
| Clinical data | |
|---|---|
| Trade names | Sovaldi, Soforal, others [1] |
| Other names | PSI-7977; GS-7977 |
| AHFS/ Drugs.com | Monograph |
| MedlinePlus | a614014 |
| License data |
|
|
Pregnancy category |
|
|
Routes of administration | by mouth [3] |
| Drug class | HCV polymerase inhibitor |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 92% |
| Protein binding | 61–65% |
| Metabolism | Quickly activated to triphosphate ( CatA/ CES1, HIST1, phosphorylation) |
| Elimination half-life | 0.4 hrs (sofosbuvir) 27 hrs (inactive metabolite GS-331007) |
| Excretion | 80% urine, 14% feces (mostly as GS-331007) |
| Identifiers | |
| |
| Chemical and physical data | |
| Formula | C22H29FN3O9P |
| Molar mass | 529.458 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
Sofosbuvir, sold under the brand name Sovaldi among others, is a medication used to treat hepatitis C. [3] It is only recommended with some combination of ribavirin, peginterferon-alfa, simeprevir, ledipasvir, daclatasvir, or velpatasvir. [7] [8] Cure rates are 30 to 97% depending on the type of hepatitis C virus involved. [9] Safety during pregnancy is unclear; some of the medications used in combination may result in harm to the baby. [9] It is taken by mouth. [3]
Common side effects include feeling tired, headache, nausea, and trouble sleeping. [3] Side effects are generally more common in interferon-containing regimens. [10]: 7 Sofosbuvir may reactivate hepatitis B in those who have been previously infected. [11] In combination with ledipasvir, daclatasvir or simeprevir it is not recommended with amiodarone due to the risk of an abnormally slow heartbeat. [10] Sofosbuvir is in the nucleotide analog family of medication and works by blocking the hepatitis C NS5B protein. [12]
Sofosbuvir was discovered in 2007, and approved for medical use in the United States in 2013. [10] [7] [13] It is on the World Health Organization's List of Essential Medicines. [14] As of 2016 [update], a 12-week course of treatment costs about US$84,000 in the United States, US$53,000 in the United Kingdom, US$45,000 in Canada, and about US$500 in India. [15] Over 60,000 people were treated with sofosbuvir in its first 30 weeks being sold in the United States. [16] The patent is not recognized by Egypt, were generic versions are made. [17]
- ^ Divya Rajagopal for the Economic Times. Sept 12, 2015. Can Indian generic makers find gold with a blockbuster Hepatitis C drug? Archived 20 March 2016 at the Wayback Machine
- ^ a b "Sofosbuvir (Sovaldi) Use During Pregnancy". Drugs.com. 16 December 2019. Archived from the original on 5 February 2020. Retrieved 5 February 2020.
- ^ a b c d e "Sofosbuvir". The American Society of Health-System Pharmacists. Archived from the original on 2016-12-01. Retrieved November 30, 2016.
- ^ "Sovaldi 400 mg film coated tablets - Summary of Product Characteristics (SmPC)". (emc). 6 November 2019. Archived from the original on 22 October 2020. Retrieved 18 April 2020.
- ^ a b "SOFOSBUVIR = SOF oral - Essential drugs". medicalguidelines.msf.org. Archived from the original on 26 November 2020. Retrieved 25 August 2020.
- ^ "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 29 July 2020. Retrieved 15 September 2020.
- ^ a b "Sofosbuvir (Sovaldi) - Treatment - Hepatitis C Online". www.hepatitisc.uw.edu. Archived from the original on 23 December 2016. Retrieved 8 January 2017.
-
^ FDA, News Release (June 28, 2016).
"FDA approves Epclusa for treatment of chronic Hepatitis C virus infection".
Archived from the original on June 3, 2017.
{{ cite journal}}: Cite journal requires|journal=( help) - ^ a b "Sovaldi 400 mg film coated tablets - Summary of Product Characteristics (SPC) - (eMC)". www.medicines.org.uk. Archived from the original on 31 January 2017. Retrieved 8 January 2017.
- ^ a b c "Sovaldi- sofosbuvir tablet, film coated Sovaldi- sofosbuvir pellet". DailyMed. 27 September 2019. Archived from the original on 21 September 2020. Retrieved 4 February 2020.
- ^ "Direct-Acting Antivirals for Hepatitis C: Drug Safety Communication - Risk of Hepatitis B Reactivating". FDA. 4 October 2016. Archived from the original on 6 October 2016. Retrieved 6 October 2016.
- ^ "Sovaldi 400 mg film coated tablets - Summary of Product Characteristics". UK Electronic Medicines Compendium. September 2016. Archived from the original on 10 November 2016. Retrieved 10 November 2016.
- ^ Gounder, Celine (9 December 2013). "A Better Treatment for Hepatitis C". The New Yorker. Archived from the original on 20 September 2016.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl: 10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Hill A, Simmons B, Gotham D, Fortunak J (January 2016). "Rapid reductions in prices for generic sofosbuvir and daclatasvir to treat hepatitis C". Journal of Virus Eradication. 2 (1): 28–31. PMC 4946692. PMID 27482432.
- ^ Alonso-Zaldivar, Ricardo (July 29, 2014). "Gilead's Sovaldi prescribed more than all other hepatitis C drugs combined". Mercury News. Archived from the original on May 23, 2015.
- ^ Radu, Alexandra (28 Jul 2021). "Affordable hepatitis drug offers new hope to millions". www.aljazeera.com. Archived from the original on 1 June 2023. Retrieved 10 September 2023.