 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Singulair, others |
| AHFS/ Drugs.com | Monograph |
| MedlinePlus | a600014 |
| License data |
|
|
Pregnancy category |
|
|
Routes of administration | By mouth |
| Drug class | Leukotriene receptor antagonist |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 63–73% |
| Protein binding | 99% |
| Metabolism | Liver ( CYP2C8-major, CYP3A4 and CYP2C9-minor) [2] |
| Elimination half-life | 2.7–5.5 hours |
| Excretion | Biliary |
| Identifiers | |
| |
| Chemical and physical data | |
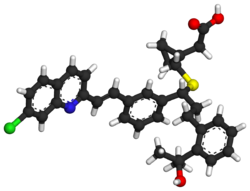
| Formula | C35H36ClNO3S |
| Molar mass | 586.19 g·mol−1 |
| 3D model ( JSmol) | |
| Melting point | 145 to 148 °C (293 to 298 °F) |
| |
| |
| (verify) | |
Montelukast, sold under the brand name Singulair among others, is a medication used in the maintenance treatment of asthma. [3] It is generally less preferred for this use than inhaled corticosteroids. [3] It is not useful for acute asthma attacks. [3] Other uses include allergic rhinitis and hives of long duration. [3] For allergic rhinitis it is a second line treatment. [5] It is taken by mouth. [3]
Common side effects include abdominal pain, cough, and headache. [3] Severe side effects may include allergic reactions, such as anaphylaxis and eosinophilia. [3] Use in pregnancy appears to be safe. [3] Montelukast is in the leukotriene receptor antagonist family of medications. [3] It works by blocking the action of leukotriene D4 in the lungs resulting in decreased inflammation and relaxation of smooth muscle. [3]
Montelukast was approved for medical use in the United States in 1998. [3] It is available as a generic medication. [6] In the United States, the wholesale cost per dose is less than 0.15 USD as of 2018. [7] In 2017, it was the 16th most commonly prescribed medication in the United States, with more than 31 million prescriptions. [8] [9]
References
- ^ a b "Montelukast (Singulair) Use During Pregnancy". Drugs.com. 13 December 2019. Archived from the original on 7 August 2019. Retrieved 4 March 2020.
- ^ "Montelukast 10 mg film coated tablets - Summary of Product Characteristics (SmPC) - (eMC)". Archived from the original on 23 December 2018. Retrieved 23 December 2018.
- ^ a b c d e f g h i j k l m "Montelukast Sodium Monograph for Professionals". Drugs.com. AHFS. Archived from the original on 7 June 2019. Retrieved 23 December 2018.
-
^ Cite error: The named reference
WHO2020DDDwas invoked but never defined (see the help page). -
^ Grainger, J.; Drake-Lee, A. (2006). "Montelukast in allergic rhinitis: a systematic review and meta-analysis". Clinical Otolaryngology. 31 (5). Wiley: 360–367.
doi:
10.1111/j.1749-4486.2006.01276.x.
ISSN
0307-7772.
PMID
17014443.
regarded as second line therapy. When used, montelukast should be used in combination with an antihistamine.
- ^ British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 269. ISBN 9780857113382.
- ^ "NADAC as of 2018-12-19". Centers for Medicare and Medicaid Services. Archived from the original on 19 December 2018. Retrieved 22 December 2018.
- ^ "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 4 March 2020.
- ^ "Montelukast - Drug Usage Statistics". ClinCalc. 23 December 2019. Archived from the original on 11 April 2020. Retrieved 11 April 2020.
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Singulair, others |
| AHFS/ Drugs.com | Monograph |
| MedlinePlus | a600014 |
| License data |
|
|
Pregnancy category |
|
|
Routes of administration | By mouth |
| Drug class | Leukotriene receptor antagonist |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 63–73% |
| Protein binding | 99% |
| Metabolism | Liver ( CYP2C8-major, CYP3A4 and CYP2C9-minor) [2] |
| Elimination half-life | 2.7–5.5 hours |
| Excretion | Biliary |
| Identifiers | |
| |
| Chemical and physical data | |
| Formula | C35H36ClNO3S |
| Molar mass | 586.19 g·mol−1 |
| 3D model ( JSmol) | |
| Melting point | 145 to 148 °C (293 to 298 °F) |
| |
| |
| (verify) | |
Montelukast, sold under the brand name Singulair among others, is a medication used in the maintenance treatment of asthma. [3] It is generally less preferred for this use than inhaled corticosteroids. [3] It is not useful for acute asthma attacks. [3] Other uses include allergic rhinitis and hives of long duration. [3] For allergic rhinitis it is a second line treatment. [5] It is taken by mouth. [3]
Common side effects include abdominal pain, cough, and headache. [3] Severe side effects may include allergic reactions, such as anaphylaxis and eosinophilia. [3] Use in pregnancy appears to be safe. [3] Montelukast is in the leukotriene receptor antagonist family of medications. [3] It works by blocking the action of leukotriene D4 in the lungs resulting in decreased inflammation and relaxation of smooth muscle. [3]
Montelukast was approved for medical use in the United States in 1998. [3] It is available as a generic medication. [6] In the United States, the wholesale cost per dose is less than 0.15 USD as of 2018. [7] In 2017, it was the 16th most commonly prescribed medication in the United States, with more than 31 million prescriptions. [8] [9]
References
- ^ a b "Montelukast (Singulair) Use During Pregnancy". Drugs.com. 13 December 2019. Archived from the original on 7 August 2019. Retrieved 4 March 2020.
- ^ "Montelukast 10 mg film coated tablets - Summary of Product Characteristics (SmPC) - (eMC)". Archived from the original on 23 December 2018. Retrieved 23 December 2018.
- ^ a b c d e f g h i j k l m "Montelukast Sodium Monograph for Professionals". Drugs.com. AHFS. Archived from the original on 7 June 2019. Retrieved 23 December 2018.
-
^ Cite error: The named reference
WHO2020DDDwas invoked but never defined (see the help page). -
^ Grainger, J.; Drake-Lee, A. (2006). "Montelukast in allergic rhinitis: a systematic review and meta-analysis". Clinical Otolaryngology. 31 (5). Wiley: 360–367.
doi:
10.1111/j.1749-4486.2006.01276.x.
ISSN
0307-7772.
PMID
17014443.
regarded as second line therapy. When used, montelukast should be used in combination with an antihistamine.
- ^ British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 269. ISBN 9780857113382.
- ^ "NADAC as of 2018-12-19". Centers for Medicare and Medicaid Services. Archived from the original on 19 December 2018. Retrieved 22 December 2018.
- ^ "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 4 March 2020.
- ^ "Montelukast - Drug Usage Statistics". ClinCalc. 23 December 2019. Archived from the original on 11 April 2020. Retrieved 11 April 2020.