 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ljʊəˈræsɪdoʊn/ |
| Trade names | Latuda, others |
| Other names | SM-13496 |
| AHFS/ Drugs.com | Monograph |
| MedlinePlus | a611016 |
| License data |
|
|
Pregnancy category |
|
|
Routes of administration | By mouth |
| Drug class | Atypical antipsychotic [2] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 9–19% (oral) [4] |
| Protein binding | ~99% [5] |
| Metabolism | Liver ( CYP3A4-mediated) [4] |
| Elimination half-life | 18–40 hours [4] [5] |
| Excretion | Faecal (67–80%), renal (9–19%) [4] [5] |
| Identifiers | |
| |
| Chemical and physical data | |
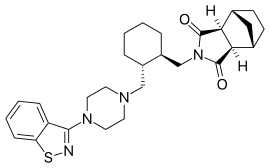
| Formula | C28H36N4O2S |
| Molar mass | 492.68 g·mol−1 |
| 3D model ( JSmol) | |
| Specific rotation | [α]20D −59° |
| Melting point | 176 to 178 °C (349 to 352 °F) |
| Solubility in water | 0.224 |
| |
| |
Lurasidone, sold under the trade name Latuda among others, is an antipsychotic medication used to treat schizophrenia and bipolar disorder. [2] In bipolar it may be used together with a mood stabilizer such as lithium or valproate. [2] It is taken by mouth. [2]
Common side effects include sleepiness, movement disorders, nausea, and diarrhea. [2] Serious side effects may include the potentially permanent movement disorder tardive dyskinesia, as well as neuroleptic malignant syndrome, an increased risk of suicide, angioedema, and high blood sugar levels. [2] In older people with psychosis as a result of dementia, it may increase the risk of dying. [2] Use during pregnancy is of unclear safety. [7] How it works is not clear but is believed to involve effects on dopamine and serotonin in the brain. [2]
Lurasidone was approved for medical use in the United States in 2010. [2] A month supply in the United Kingdom costs the NHS about £91 as of 2019. [7] In the United States this amount is about US$1,350 as of 2020. [8] In 2019 generic versions were approved in the United States but will not be available until 2023. [9] [10] In 2017, it was the 274th most commonly prescribed medication in the United States, with more than one million prescriptions. [11] [12]
- ^ a b "Lurasidone (Latuda) Use During Pregnancy". Drugs.com. 5 February 2020. Archived from the original on 15 October 2020. Retrieved 12 May 2020.
- ^ a b c d e f g h i j k "Lurasidone Hydrochloride Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 21 March 2019. Retrieved 21 March 2019.
- ^ "Latuda 18.5mg film-coated tablets - Summary of Product Characteristics (SmPC)". (emc). 16 January 2019. Archived from the original on 14 October 2020. Retrieved 12 May 2020.
- ^ a b c d "Product information Latuda (lurasidone hydrochloride)" (PDF). TGA eBusiness Services. Therapeutic Goods Administration. 16 April 2014. Archived from the original on 18 October 2018. Retrieved 1 May 2014.
- ^ a b c "Latuda: EPAR – Product Information" (PDF). European Medicines Agency. 14 April 2016. Archived (PDF) from the original on 21 August 2016. Retrieved 27 February 2017.
-
^ Cite error: The named reference
WHO2020DDDwas invoked but never defined (see the help page). - ^ a b British national formulary: BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 393–394. ISBN 9780857113382.
- ^ "Latuda Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 13 August 2020. Retrieved 30 April 2020.
- ^ "Generic Latuda Availability". Drugs.com. Archived from the original on 14 August 2020. Retrieved 30 April 2020.
-
^ Cite error: The named reference
WSJ2019was invoked but never defined (see the help page). - ^ "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- ^ "Lurasidone Hydrochloride - Drug Usage Statistics". ClinCalc. Archived from the original on 8 July 2020. Retrieved 11 April 2020.
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ljʊəˈræsɪdoʊn/ |
| Trade names | Latuda, others |
| Other names | SM-13496 |
| AHFS/ Drugs.com | Monograph |
| MedlinePlus | a611016 |
| License data |
|
|
Pregnancy category |
|
|
Routes of administration | By mouth |
| Drug class | Atypical antipsychotic [2] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 9–19% (oral) [4] |
| Protein binding | ~99% [5] |
| Metabolism | Liver ( CYP3A4-mediated) [4] |
| Elimination half-life | 18–40 hours [4] [5] |
| Excretion | Faecal (67–80%), renal (9–19%) [4] [5] |
| Identifiers | |
| |
| Chemical and physical data | |
| Formula | C28H36N4O2S |
| Molar mass | 492.68 g·mol−1 |
| 3D model ( JSmol) | |
| Specific rotation | [α]20D −59° |
| Melting point | 176 to 178 °C (349 to 352 °F) |
| Solubility in water | 0.224 |
| |
| |
Lurasidone, sold under the trade name Latuda among others, is an antipsychotic medication used to treat schizophrenia and bipolar disorder. [2] In bipolar it may be used together with a mood stabilizer such as lithium or valproate. [2] It is taken by mouth. [2]
Common side effects include sleepiness, movement disorders, nausea, and diarrhea. [2] Serious side effects may include the potentially permanent movement disorder tardive dyskinesia, as well as neuroleptic malignant syndrome, an increased risk of suicide, angioedema, and high blood sugar levels. [2] In older people with psychosis as a result of dementia, it may increase the risk of dying. [2] Use during pregnancy is of unclear safety. [7] How it works is not clear but is believed to involve effects on dopamine and serotonin in the brain. [2]
Lurasidone was approved for medical use in the United States in 2010. [2] A month supply in the United Kingdom costs the NHS about £91 as of 2019. [7] In the United States this amount is about US$1,350 as of 2020. [8] In 2019 generic versions were approved in the United States but will not be available until 2023. [9] [10] In 2017, it was the 274th most commonly prescribed medication in the United States, with more than one million prescriptions. [11] [12]
- ^ a b "Lurasidone (Latuda) Use During Pregnancy". Drugs.com. 5 February 2020. Archived from the original on 15 October 2020. Retrieved 12 May 2020.
- ^ a b c d e f g h i j k "Lurasidone Hydrochloride Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 21 March 2019. Retrieved 21 March 2019.
- ^ "Latuda 18.5mg film-coated tablets - Summary of Product Characteristics (SmPC)". (emc). 16 January 2019. Archived from the original on 14 October 2020. Retrieved 12 May 2020.
- ^ a b c d "Product information Latuda (lurasidone hydrochloride)" (PDF). TGA eBusiness Services. Therapeutic Goods Administration. 16 April 2014. Archived from the original on 18 October 2018. Retrieved 1 May 2014.
- ^ a b c "Latuda: EPAR – Product Information" (PDF). European Medicines Agency. 14 April 2016. Archived (PDF) from the original on 21 August 2016. Retrieved 27 February 2017.
-
^ Cite error: The named reference
WHO2020DDDwas invoked but never defined (see the help page). - ^ a b British national formulary: BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 393–394. ISBN 9780857113382.
- ^ "Latuda Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 13 August 2020. Retrieved 30 April 2020.
- ^ "Generic Latuda Availability". Drugs.com. Archived from the original on 14 August 2020. Retrieved 30 April 2020.
-
^ Cite error: The named reference
WSJ2019was invoked but never defined (see the help page). - ^ "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- ^ "Lurasidone Hydrochloride - Drug Usage Statistics". ClinCalc. Archived from the original on 8 July 2020. Retrieved 11 April 2020.