 | |
| Clinical data | |
|---|---|
| Trade names | Nozinan, Levoprome, Detenler, Hirnamin, Levotomin, Neurocil, others |
| AHFS/ Drugs.com | International Drug Names |
|
Pregnancy category |
|
|
Routes of administration | By mouth, IV, SC, IM [1] |
| Drug class | Typical antipsychotic |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~50–60% |
| Metabolism | Liver |
| Onset of action | 0.5 to 3 hr [2] |
| Elimination half-life | ~20 hours |
| Duration of action | 8 hr [2] |
| Excretion | In feces and urine (metabolites), unchanged drug only 1% |
| Identifiers | |
| |
| Chemical and physical data | |
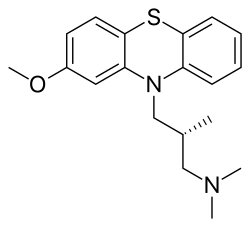
| Formula | C19H24N2OS |
| Molar mass | 328.47 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
| | |
Levomepromazine, also known as methotrimeprazine, is a medication used for schizophrenia and palliative care. [1] In palliative care it is used for restlessness, pain, and vomiting. [1] It may be used by mouth or by injection. [1] Effects generally begin around 0.5 to 3 hours and last for 8 hours. [2]
Side effects may include low blood pressure with standing, sleepiness, dry mouth, liver problems, and dystonia. [2] [1] Serious side effect may include priapism, QT prolongation, and neuroleptic malignant syndrome. [2] [1] [3] It is an antipsychotic of the phenothiazine type. [2] It works by blocking a variety of receptors, including adrenergic, dopamine, histamine, muscarinic acetylcholine, and serotonin. [2]
Levomepromazine was patented in 1954 and come into medical use in the United State in 1957. [4] It is available as a generic medication. [1] In the United Kingdom 84 tablets of 25 mg costs the NHS about £20 as of 2020. [1] It has been widely used; [2] though is no longer commercially available in the United States. [5]
References
- ^
a
b
c
d
e
f
g
h
i
j BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 460.
ISBN
978-0-85711-369-6.
{{ cite book}}: CS1 maint: date format ( link) - ^
a
b
c
d
e
f
g
h
i Dietz, I; Schmitz, A; Lampey, I; Schulz, C (19 January 2013). "Evidence for the use of Levomepromazine for symptom control in the palliative care setting: a systematic review". BMC palliative care. 12: 2.
doi:
10.1186/1472-684X-12-2.
PMID
23331515.
{{ cite journal}}: CS1 maint: unflagged free DOI ( link) - ^ "Levomepromazine Hydrochloride 25mg/ml Solution for Injection - Summary of Product Characteristics (SmPC) - (emc)". www.medicines.org.uk. Archived from the original on 28 August 2021. Retrieved 13 August 2021.
- ^ Buschmann, Helmut; Holenz, Jörg; Párraga, Antonio; Torrens, Antoni; Vela, José Miguel; Díaz, José Luis (16 April 2007). Antidepressants, Antipsychotics, Anxiolytics, 2 Volume Set: From Chemistry and Pharmacology to Clinical Application. John Wiley & Sons. p. 502. ISBN 978-3-527-31058-6. Archived from the original on 28 August 2021. Retrieved 13 August 2021.
- ^ "Drugs@FDA: FDA-Approved Drugs". www.accessdata.fda.gov. Archived from the original on 24 March 2021. Retrieved 13 August 2021.
 | |
| Clinical data | |
|---|---|
| Trade names | Nozinan, Levoprome, Detenler, Hirnamin, Levotomin, Neurocil, others |
| AHFS/ Drugs.com | International Drug Names |
|
Pregnancy category |
|
|
Routes of administration | By mouth, IV, SC, IM [1] |
| Drug class | Typical antipsychotic |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~50–60% |
| Metabolism | Liver |
| Onset of action | 0.5 to 3 hr [2] |
| Elimination half-life | ~20 hours |
| Duration of action | 8 hr [2] |
| Excretion | In feces and urine (metabolites), unchanged drug only 1% |
| Identifiers | |
| |
| Chemical and physical data | |
| Formula | C19H24N2OS |
| Molar mass | 328.47 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
| | |
Levomepromazine, also known as methotrimeprazine, is a medication used for schizophrenia and palliative care. [1] In palliative care it is used for restlessness, pain, and vomiting. [1] It may be used by mouth or by injection. [1] Effects generally begin around 0.5 to 3 hours and last for 8 hours. [2]
Side effects may include low blood pressure with standing, sleepiness, dry mouth, liver problems, and dystonia. [2] [1] Serious side effect may include priapism, QT prolongation, and neuroleptic malignant syndrome. [2] [1] [3] It is an antipsychotic of the phenothiazine type. [2] It works by blocking a variety of receptors, including adrenergic, dopamine, histamine, muscarinic acetylcholine, and serotonin. [2]
Levomepromazine was patented in 1954 and come into medical use in the United State in 1957. [4] It is available as a generic medication. [1] In the United Kingdom 84 tablets of 25 mg costs the NHS about £20 as of 2020. [1] It has been widely used; [2] though is no longer commercially available in the United States. [5]
References
- ^
a
b
c
d
e
f
g
h
i
j BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 460.
ISBN
978-0-85711-369-6.
{{ cite book}}: CS1 maint: date format ( link) - ^
a
b
c
d
e
f
g
h
i Dietz, I; Schmitz, A; Lampey, I; Schulz, C (19 January 2013). "Evidence for the use of Levomepromazine for symptom control in the palliative care setting: a systematic review". BMC palliative care. 12: 2.
doi:
10.1186/1472-684X-12-2.
PMID
23331515.
{{ cite journal}}: CS1 maint: unflagged free DOI ( link) - ^ "Levomepromazine Hydrochloride 25mg/ml Solution for Injection - Summary of Product Characteristics (SmPC) - (emc)". www.medicines.org.uk. Archived from the original on 28 August 2021. Retrieved 13 August 2021.
- ^ Buschmann, Helmut; Holenz, Jörg; Párraga, Antonio; Torrens, Antoni; Vela, José Miguel; Díaz, José Luis (16 April 2007). Antidepressants, Antipsychotics, Anxiolytics, 2 Volume Set: From Chemistry and Pharmacology to Clinical Application. John Wiley & Sons. p. 502. ISBN 978-3-527-31058-6. Archived from the original on 28 August 2021. Retrieved 13 August 2021.
- ^ "Drugs@FDA: FDA-Approved Drugs". www.accessdata.fda.gov. Archived from the original on 24 March 2021. Retrieved 13 August 2021.