 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | del a' vir deen [1] |
| Trade names | Rescriptor |
| Other names | Delavirdine mesylate |
| AHFS/ Drugs.com | Monograph |
| MedlinePlus | a600034 |
|
Pregnancy category |
|
|
Routes of administration | By mouth |
| Drug class | Non-nucleoside reverse transcriptase inhibitor (NNRTI) [2] |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 85% |
| Protein binding | 98% |
| Metabolism | Liver ( CYP3A4- and CYP2D6-mediated) |
| Elimination half-life | 5.8 hours |
| Excretion | Kidney (51%) and fecal (44%) |
| Identifiers | |
| |
| Chemical and physical data | |
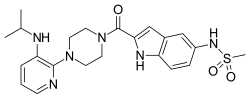
| Formula | C22H28N6O3S |
| Molar mass | 456.57 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
| (verify) | |
Delavirdine (DLV), sold under the brand name Rescriptor, is a medication used to treat HIV/AIDS. [2] It is used together with other HIV medicines; though is not a preferred treatment. [2] It is taken by mouth, three times per day. [2]
Common side effects include tiredness, dizziness, headache, and rash. [1] Other symptoms may include Stevens-Johnson syndrome, central obesity, and immune reconstitution syndrome. [2] Safety in pregnancy is unclear. [2] It is a non-nucleoside reverse transcriptase inhibitor (NNRTI). [2]
Delavirdine was approved for medical use in the United States in 1997. [2] It has been discontinued in the United States as of 2021. [2] It is not commonly used. [1]
References
- ^ a b c d "Delavirdine". LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases. 2012. Archived from the original on 6 May 2021. Retrieved 23 December 2021.
- ^ a b c d e f g h i j k "Delavirdine Mesylate Monograph for Professionals". Drugs.com. Archived from the original on 15 May 2016. Retrieved 23 December 2021.
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | del a' vir deen [1] |
| Trade names | Rescriptor |
| Other names | Delavirdine mesylate |
| AHFS/ Drugs.com | Monograph |
| MedlinePlus | a600034 |
|
Pregnancy category |
|
|
Routes of administration | By mouth |
| Drug class | Non-nucleoside reverse transcriptase inhibitor (NNRTI) [2] |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 85% |
| Protein binding | 98% |
| Metabolism | Liver ( CYP3A4- and CYP2D6-mediated) |
| Elimination half-life | 5.8 hours |
| Excretion | Kidney (51%) and fecal (44%) |
| Identifiers | |
| |
| Chemical and physical data | |
| Formula | C22H28N6O3S |
| Molar mass | 456.57 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
| (verify) | |
Delavirdine (DLV), sold under the brand name Rescriptor, is a medication used to treat HIV/AIDS. [2] It is used together with other HIV medicines; though is not a preferred treatment. [2] It is taken by mouth, three times per day. [2]
Common side effects include tiredness, dizziness, headache, and rash. [1] Other symptoms may include Stevens-Johnson syndrome, central obesity, and immune reconstitution syndrome. [2] Safety in pregnancy is unclear. [2] It is a non-nucleoside reverse transcriptase inhibitor (NNRTI). [2]
Delavirdine was approved for medical use in the United States in 1997. [2] It has been discontinued in the United States as of 2021. [2] It is not commonly used. [1]
References
- ^ a b c d "Delavirdine". LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases. 2012. Archived from the original on 6 May 2021. Retrieved 23 December 2021.
- ^ a b c d e f g h i j k "Delavirdine Mesylate Monograph for Professionals". Drugs.com. Archived from the original on 15 May 2016. Retrieved 23 December 2021.