| |||
| Names | |||
|---|---|---|---|
|
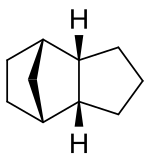
IUPAC name
tricyclo[5.2.1.02,6]decane
| |||
| Other names
Tetrahydrodicyclopentadiene
| |||
| Identifiers | |||
3D model (
JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
PubChem
CID
|
|||
CompTox Dashboard (
EPA)
|
|||
| |||
| |||
| Properties | |||
| C10H16 | |||
| Molar mass | 136.238 g·mol−1 | ||
| Hazards | |||
| GHS labelling: [1] | |||



| |||
| Danger | |||
| H226, H302, H304, H315, H319, H335 | |||
| P210, P233, P240, P241, P242, P243, P261, P264, P264+P265, P270, P271, P280, P301+P316, P301+P317, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P319, P321, P330, P331, P332+P317, P337+P317, P362+P364, P370+P378, P403+P233, P403+P235, P405, P501 | |||
| Related compounds | |||
Related compounds
|
Twistane | ||
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |||
Tricyclodecane (TCD) is an organic compound with the formula C10H16. It is classed as a hydrocarbon. It has two main stereoisomers–the endo and exo forms. [2] Its primary use in the exo form is as a component of jet fuel. [3] It is used here primarily because of its high energy density. The exo isomer also has a low freezing point. [4] [5] Because of this, its properties have been studied extensively. [6] [7] [8] [9] [10] It is often called tetrahydro dicyclopentadiene.
Its reactions with other materials has been studied, [11] [12] as have various production methods. [13] [14] The two isomers can interconvert in the presence of aluminum chloride as catalyst absorbed on substrates such as silicon dioxide or zeolites, [15] [16] [17] [18] with preference for forming the exo as the major product. [19] [20]
- ^ "Tetrahydrodicyclopentadiene". pubchem.ncbi.nlm.nih.gov.
- ^ "Exo-tricyclo[5.2.1.0(2.6)]decane". webbook.nist.gov. Retrieved 2023-10-03.
- ^ Hudzik, Jason M.; Asatryan, Rubik; Bozzelli, Joseph W. (2010-09-09). "Thermochemical Properties of exo -Tricyclo[5.2.1.0 2,6 ]decane (JP-10 Jet Fuel) and Derived Tricyclodecyl Radicals". The Journal of Physical Chemistry A. 114 (35): 9545–9553. Bibcode: 2010JPCA..114.9545H. doi: 10.1021/jp1049556. ISSN 1089-5639. PMID 20712369.
- ^ Herbinet, Olivier; Sirjean, Baptiste; Bounaceur, Roda; Fournet, René; Battin-Leclerc, Frédérique; Scacchi, Gérard; Marquaire, Paul-Marie (2006-10-01). "Primary Mechanism of the Thermal Decomposition of Tricyclodecane". The Journal of Physical Chemistry A. 110 (39): 11298–11314. Bibcode: 2006JPCA..11011298H. doi: 10.1021/jp0623802. ISSN 1089-5639. PMID 17004739.
- ^ Wu, Junjun; Gao, Lu Gem; Ning, Hongbo; Ren, Wei; Truhlar, Donald G. (2020-06-01). "Direct dynamics of a large complex hydrocarbon reaction system: The reaction of OH with exo-tricyclodecane (the main component of Jet Propellant-10)". Combustion and Flame. 216: 82–91. Bibcode: 2020CoFl..216...82W. doi: 10.1016/j.combustflame.2020.02.019. ISSN 0010-2180. S2CID 216384271.
- ^ "Exo-tricyclo[5.2.1.0(2.6)]decane". Cheméo. Retrieved 2023-10-03.
- ^ Seiser, R.; Niemann, U.; Seshadri, K. (2011-01-01). "Experimental study of combustion of n-decane and JP-10 in non-premixed flows". Proceedings of the Combustion Institute. 33 (1): 1045–1052. doi: 10.1016/j.proci.2010.06.078. ISSN 1540-7489.
- ^ Tao, Yujie; Xu, Rui; Wang, Kun; Shao, Jiankun; Johnson, Sarah E.; Movaghar, Ashkan; Han, Xu; Park, Ji-Woong; Lu, Tianfeng; Brezinsky, Kenneth; Egolfopoulos, Fokion N.; Davidson, David F.; Hanson, Ronald K.; Bowman, Craig T.; Wang, Hai (2018-12-01). "A Physics based approach to modeling real fuel combustion chemistry III Reaction kinetic model of JP10". Combustion and Flame. 198: 466–476. Bibcode: 2018CoFl..198..466T. doi: 10.1016/j.combustflame.2018.08.022. ISSN 0010-2180. S2CID 104745782.
- ^ Li, Heng; Liu, Guozhu; Jiang, Rongpei; Wang, Li; Zhang, Xiangwen (2015-05-01). "Experimental and kinetic modeling study of exo-TCD pyrolysis under low pressure". Combustion and Flame. 162 (5): 2177–2190. Bibcode: 2015CoFl..162.2177L. doi: 10.1016/j.combustflame.2015.01.015. ISSN 0010-2180.
- ^ Goh, K. H. H.; Geipel, P.; Hampp, F.; Lindstedt, R. P. (2013-01-01). "Regime transition from premixed to flameless oxidation in turbulent JP-10 flames". Proceedings of the Combustion Institute. 34 (2): 3311–3318. doi: 10.1016/j.proci.2012.06.173. ISSN 1540-7489.
- ^ "STTR Navy FY09A - Characterization of the High Temperature Decomposition Products of JP-10". www.navysbir.com. Retrieved 2023-10-03.
- ^ Wu, Junjun; Gao, Lu Gem; Ning, Hongbo; Ren, Wei; Truhlar, Donald G. (2020-06-01). "Direct dynamics of a large complex hydrocarbon reaction system: The reaction of OH with exo-tricyclodecane (the main component of Jet Propellant-10)". Combustion and Flame. 216: 82–91. Bibcode: 2020CoFl..216...82W. doi: 10.1016/j.combustflame.2020.02.019. ISSN 0010-2180. S2CID 216384271.
- ^ US 2766301, Büchner, Karl; Roelen, Otto & Meis, Josef, "Production of tricyclodecane", published 1956-10-09, assigned to Ruhrchemie AG
- ^ Baptiste, Sirjean. "Theoretical Study of the Thermal Decomposition of a Jet Fuel Surrogate".
- ^ Sun, Cong-ming; Li, Gang (2011-07-31). "Vapor-phase isomerization of endo-tetrahydrodicyclopentadiene to its exo isomer over zeolite catalysts". Applied Catalysis A: General. 402 (1): 196–200. doi: 10.1016/j.apcata.2011.06.008. ISSN 0926-860X.
- ^ Campo, Pablo del; Martínez, Cristina; Corma, Avelino (2021-08-02). "Activation and conversion of alkanes in the confined space of zeolite-type materials". Chemical Society Reviews. 50 (15): 8511–8595. doi: 10.1039/D0CS01459A. hdl: 10251/183985. ISSN 1460-4744. PMID 34128513. S2CID 235437726.
- ^ Navrátilová, Markéta; Sporka, Karel (2000-09-18). "Synthesis of adamantane on commercially available zeolitic catalysts". Applied Catalysis A: General. 203 (1): 127–132. doi: 10.1016/S0926-860X(00)00477-4. ISSN 0926-860X.
- ^ Gallego, Eva María; Portilla, M. Teresa; Paris, Cecilia; León-Escamilla, Alejandro; Boronat, Mercedes; Moliner, Manuel; Corma, Avelino (2017-03-10). ""Ab initio" synthesis of zeolites for preestablished catalytic reactions". Science. 355 (6329): 1051–1054. Bibcode: 2017Sci...355.1051G. doi: 10.1126/science.aal0121. hdl: 10251/105508. ISSN 0036-8075. PMID 28280200. S2CID 206654251.
- ^ Lili, Q. I.; Min*, J. I.; Xinkui, Wang; Min, H. E.; Tianxi, C. a. I. (2010-04-25). "AlCl3/MCM-41 as a Catalyst for Isomerization of Endo-tricyclodecane". Chinese Journal of Catalysis. 31 (4): 383. ISSN 0253-9837.
- ^ Clarke, J. K. A.; Rooney, J. J. (1976-01-01), Eley, D. D.; Pines, Herman; Weisz, Paul B. (eds.), Stereochemical Approaches to Mechanisms of Hydrocarbon Reactions on Metal Catalysts, Advances in Catalysis, vol. 25, Academic Press, pp. 125–183, doi: 10.1016/s0360-0564(08)60314-4, ISBN 978-0-12-007825-7, retrieved 2023-11-20
| |||
| Names | |||
|---|---|---|---|
|
IUPAC name
tricyclo[5.2.1.02,6]decane
| |||
| Other names
Tetrahydrodicyclopentadiene
| |||
| Identifiers | |||
3D model (
JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
PubChem
CID
|
|||
CompTox Dashboard (
EPA)
|
|||
| |||
| |||
| Properties | |||
| C10H16 | |||
| Molar mass | 136.238 g·mol−1 | ||
| Hazards | |||
| GHS labelling: [1] | |||



| |||
| Danger | |||
| H226, H302, H304, H315, H319, H335 | |||
| P210, P233, P240, P241, P242, P243, P261, P264, P264+P265, P270, P271, P280, P301+P316, P301+P317, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P319, P321, P330, P331, P332+P317, P337+P317, P362+P364, P370+P378, P403+P233, P403+P235, P405, P501 | |||
| Related compounds | |||
Related compounds
|
Twistane | ||
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |||
Tricyclodecane (TCD) is an organic compound with the formula C10H16. It is classed as a hydrocarbon. It has two main stereoisomers–the endo and exo forms. [2] Its primary use in the exo form is as a component of jet fuel. [3] It is used here primarily because of its high energy density. The exo isomer also has a low freezing point. [4] [5] Because of this, its properties have been studied extensively. [6] [7] [8] [9] [10] It is often called tetrahydro dicyclopentadiene.
Its reactions with other materials has been studied, [11] [12] as have various production methods. [13] [14] The two isomers can interconvert in the presence of aluminum chloride as catalyst absorbed on substrates such as silicon dioxide or zeolites, [15] [16] [17] [18] with preference for forming the exo as the major product. [19] [20]
- ^ "Tetrahydrodicyclopentadiene". pubchem.ncbi.nlm.nih.gov.
- ^ "Exo-tricyclo[5.2.1.0(2.6)]decane". webbook.nist.gov. Retrieved 2023-10-03.
- ^ Hudzik, Jason M.; Asatryan, Rubik; Bozzelli, Joseph W. (2010-09-09). "Thermochemical Properties of exo -Tricyclo[5.2.1.0 2,6 ]decane (JP-10 Jet Fuel) and Derived Tricyclodecyl Radicals". The Journal of Physical Chemistry A. 114 (35): 9545–9553. Bibcode: 2010JPCA..114.9545H. doi: 10.1021/jp1049556. ISSN 1089-5639. PMID 20712369.
- ^ Herbinet, Olivier; Sirjean, Baptiste; Bounaceur, Roda; Fournet, René; Battin-Leclerc, Frédérique; Scacchi, Gérard; Marquaire, Paul-Marie (2006-10-01). "Primary Mechanism of the Thermal Decomposition of Tricyclodecane". The Journal of Physical Chemistry A. 110 (39): 11298–11314. Bibcode: 2006JPCA..11011298H. doi: 10.1021/jp0623802. ISSN 1089-5639. PMID 17004739.
- ^ Wu, Junjun; Gao, Lu Gem; Ning, Hongbo; Ren, Wei; Truhlar, Donald G. (2020-06-01). "Direct dynamics of a large complex hydrocarbon reaction system: The reaction of OH with exo-tricyclodecane (the main component of Jet Propellant-10)". Combustion and Flame. 216: 82–91. Bibcode: 2020CoFl..216...82W. doi: 10.1016/j.combustflame.2020.02.019. ISSN 0010-2180. S2CID 216384271.
- ^ "Exo-tricyclo[5.2.1.0(2.6)]decane". Cheméo. Retrieved 2023-10-03.
- ^ Seiser, R.; Niemann, U.; Seshadri, K. (2011-01-01). "Experimental study of combustion of n-decane and JP-10 in non-premixed flows". Proceedings of the Combustion Institute. 33 (1): 1045–1052. doi: 10.1016/j.proci.2010.06.078. ISSN 1540-7489.
- ^ Tao, Yujie; Xu, Rui; Wang, Kun; Shao, Jiankun; Johnson, Sarah E.; Movaghar, Ashkan; Han, Xu; Park, Ji-Woong; Lu, Tianfeng; Brezinsky, Kenneth; Egolfopoulos, Fokion N.; Davidson, David F.; Hanson, Ronald K.; Bowman, Craig T.; Wang, Hai (2018-12-01). "A Physics based approach to modeling real fuel combustion chemistry III Reaction kinetic model of JP10". Combustion and Flame. 198: 466–476. Bibcode: 2018CoFl..198..466T. doi: 10.1016/j.combustflame.2018.08.022. ISSN 0010-2180. S2CID 104745782.
- ^ Li, Heng; Liu, Guozhu; Jiang, Rongpei; Wang, Li; Zhang, Xiangwen (2015-05-01). "Experimental and kinetic modeling study of exo-TCD pyrolysis under low pressure". Combustion and Flame. 162 (5): 2177–2190. Bibcode: 2015CoFl..162.2177L. doi: 10.1016/j.combustflame.2015.01.015. ISSN 0010-2180.
- ^ Goh, K. H. H.; Geipel, P.; Hampp, F.; Lindstedt, R. P. (2013-01-01). "Regime transition from premixed to flameless oxidation in turbulent JP-10 flames". Proceedings of the Combustion Institute. 34 (2): 3311–3318. doi: 10.1016/j.proci.2012.06.173. ISSN 1540-7489.
- ^ "STTR Navy FY09A - Characterization of the High Temperature Decomposition Products of JP-10". www.navysbir.com. Retrieved 2023-10-03.
- ^ Wu, Junjun; Gao, Lu Gem; Ning, Hongbo; Ren, Wei; Truhlar, Donald G. (2020-06-01). "Direct dynamics of a large complex hydrocarbon reaction system: The reaction of OH with exo-tricyclodecane (the main component of Jet Propellant-10)". Combustion and Flame. 216: 82–91. Bibcode: 2020CoFl..216...82W. doi: 10.1016/j.combustflame.2020.02.019. ISSN 0010-2180. S2CID 216384271.
- ^ US 2766301, Büchner, Karl; Roelen, Otto & Meis, Josef, "Production of tricyclodecane", published 1956-10-09, assigned to Ruhrchemie AG
- ^ Baptiste, Sirjean. "Theoretical Study of the Thermal Decomposition of a Jet Fuel Surrogate".
- ^ Sun, Cong-ming; Li, Gang (2011-07-31). "Vapor-phase isomerization of endo-tetrahydrodicyclopentadiene to its exo isomer over zeolite catalysts". Applied Catalysis A: General. 402 (1): 196–200. doi: 10.1016/j.apcata.2011.06.008. ISSN 0926-860X.
- ^ Campo, Pablo del; Martínez, Cristina; Corma, Avelino (2021-08-02). "Activation and conversion of alkanes in the confined space of zeolite-type materials". Chemical Society Reviews. 50 (15): 8511–8595. doi: 10.1039/D0CS01459A. hdl: 10251/183985. ISSN 1460-4744. PMID 34128513. S2CID 235437726.
- ^ Navrátilová, Markéta; Sporka, Karel (2000-09-18). "Synthesis of adamantane on commercially available zeolitic catalysts". Applied Catalysis A: General. 203 (1): 127–132. doi: 10.1016/S0926-860X(00)00477-4. ISSN 0926-860X.
- ^ Gallego, Eva María; Portilla, M. Teresa; Paris, Cecilia; León-Escamilla, Alejandro; Boronat, Mercedes; Moliner, Manuel; Corma, Avelino (2017-03-10). ""Ab initio" synthesis of zeolites for preestablished catalytic reactions". Science. 355 (6329): 1051–1054. Bibcode: 2017Sci...355.1051G. doi: 10.1126/science.aal0121. hdl: 10251/105508. ISSN 0036-8075. PMID 28280200. S2CID 206654251.
- ^ Lili, Q. I.; Min*, J. I.; Xinkui, Wang; Min, H. E.; Tianxi, C. a. I. (2010-04-25). "AlCl3/MCM-41 as a Catalyst for Isomerization of Endo-tricyclodecane". Chinese Journal of Catalysis. 31 (4): 383. ISSN 0253-9837.
- ^ Clarke, J. K. A.; Rooney, J. J. (1976-01-01), Eley, D. D.; Pines, Herman; Weisz, Paul B. (eds.), Stereochemical Approaches to Mechanisms of Hydrocarbon Reactions on Metal Catalysts, Advances in Catalysis, vol. 25, Academic Press, pp. 125–183, doi: 10.1016/s0360-0564(08)60314-4, ISBN 978-0-12-007825-7, retrieved 2023-11-20