This article has multiple issues. Please help
improve it or discuss these issues on the
talk page. (
Learn how and when to remove these template messages)
|
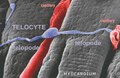
Telocytes are a type of interstitial ( stromal) cells with very long (tens to hundreds of micrometres) and very thin prolongations (mostly below the resolving power of light microscopy) called telopodes. [1]








Rationale for the term telocyte
Professor Laurențiu M. Popescu's group from Bucharest, Romania described a new type of cell. Popescu coined the terms telocytes (TC) for these cells, and telopodes (Tp) [2] for their extremely long but thin prolongations [2] [3] [4] [5] [6] [7] [8] in order to prevent further confusion with other interstitial (stromal) cells (e.g., fibroblast, fibroblast-like cells, myofibroblast, mesenchymal cells). Telopodes present an alternation of thin segments, podomeres (with caliber mostly under 200 nm, below the resolving power of light microscopy) and dilated segments, podoms, which accommodate mitochondria, (rough) endoplasmic reticulum and caveolae - the so-called "Ca2+ uptake/release units". The concept of TC was promptly adopted by other laboratories, as well. [9] [10] [11] [12] [13] [14] [15] [16] [17] [18] [19]
Telocytes and/or fibroblasts?
The interstitium ( stroma) is in most of the cases seen as a connecting "device" for the specific structures of an organ. Usually, people perceive interstitial cells as being mainly (or even, only) fibroblasts. However, fibroblasts have the function of generating connective tissue matrix, specifically, collagen. The distinction between TC and fibroblasts is obvious since they have different ultrastructure and phenotype. Therefore, their functions should be mostly different: TC - intercellular signaling (connections), but fibroblasts - collagen synthesis. In other words, TC are "more" functionally oriented while fibroblasts are "more" structurally oriented, responsible for fibrosis.
There are some clear ultrastructural features that differentiate telocytes from fibroblasts. For instance, the general aspect of TC is of a small oval ( piriform/spindle/triangular/ stellate)-shaped cellular body, containing a nucleus surrounded by a small amount of cytoplasm. Anyway, the shape of the cell body depends on the number of Tp. TC cellular body average dimensions are, as measured on EM images, 9.3 μm ± 3.2 μm (min. 6.3μm; max. 16.4 μm). Fibroblast nucleus is typically euchromatic, but TC nucleus is mostly heterochromatic. Mitochondria represent only 2% of cell body volume and the Golgi complex is small in TC. Fibroblasts Golgi complex is prominent and the rough endoplasmic reticulum is very well developed (usually 5-12%) of cell volume.
Since telopodes are distinctive for telocytes, here are their main features:
- Number: 1–5 (frequently only 2–3 telopodes are observed on a single section, depending on site and angle of section, since their 3D convolutions prevent them to be observed at their full length in a 2D very thin section);
- Length: tens – up to hundreds of μm, as measured on EM images (e.g. Figs. 2-10). However, under favorable conditions in cell cultures, their entire length can be captured in several successive images (Fig. 1);
- Thickness: uneven caliber, mostly below 0.2 μm (below the resolving power of light microscopy), visible under electron microscopy;
- Moniliform aspect: podoms and podomeres; average caliber of podomeres: 0.1 μm ± 0.05μm, min. = 0.003 μm; max. = 0.24 μm; Podoms accommodate: mitochondria, (rough) endoplasmic reticulum, caveolae, a trio called ‘Ca2+-uptake/release units’.
- Branching, with a dichotomous pattern;
- Organization in a labyrinthine system, forming a 3D network anchored by hetero- and homocellular junctions.
Summary
Here is shown visual evidence (electron microscopy, electron tomography, phase contrast microscopy) for the existence of Telocytes (TC) in many organs from human and rodents. TC and Tp, and also podoms and podomeres were found in:
- cavitary organs:
- heart ( endo-, myo-, and pericardium);
- stomach and intestine, with mesentery;
- gall bladder;
- uterus and fallopian tube; [20] [21]
- non-cavitary organs:
- lungs and pleura; [8] [22] [23]
- pancreas ( exocrine gland); [24]
- mammary gland;
- placenta; [3]
- kidneys; [25] [26]
Recent evidence shows the involvement of TC in pathology. [27] TC are strategically located in between blood vessels ( capillaries), nerve endings and the specific resident cell populations of a given organ. TC establish via Tp homo- and heterocellular junctions and release shed vesicles and exosomes.
Perspectives: regenerative medicine
TC and SC make a tandem (due to specific intercellular junctions) within the so-called SC niches, at least in heart [28] and lungs. Hence, TC could be key-players in regenerating and repair of some organs. The tandem TC-SC could be a better option for therapy rather than SC alone. Published studies suggest that cardiac TCs could be regarded as a potential cell source for therapeutic use to improve cardiac repair and function after a myocardial infarction, either alone or in tandem with SC. [29] Recent data show that TCs are completely different from FBs, using a quantitative proteomics approach, suggesting that TCs might play specific roles in mechanical sensing and mechanochemical conversion task, tissue homoeostasis and remodelling/renewal. [23]
Figures
-
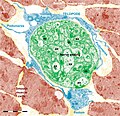
Figure 9. Human mammary gland stroma: TEM; original magnification 9,100x. A: Lymphocyte establishing a multicontact synapse (MS) with a TC. The blue rectangle shows the synaptic ‘kiss and run’ region. The synaptic membranes appear traced in B (violet - TC, orange - lymphocyte). The distances between membranes are shown in C. Note (asterisk) a peculiar conformation of ER connecting mitochondria with the cell surface, suggestive for a possible role in synaptic Ca2+ homeostasis. Reproduced with permission from [22]
-
Figure 10. Scanning electron micrograph of monkey left ventricular myocardium. A typical TC is located across the cardiomyocytes, in close contacts with blood capillaries. Note, the cardiomyocytes striations and the openings of T tubules.
-
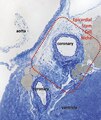
Figure 11. Digitally coloured electron micrograph of mouse ventricular endocardium (burgundy). TC (blue) make an interstitial network in the heart. Subendocardial telocytes (TC1) sends Tp between cardiomyocytes (CM) and communicate with TC2. Cap, blood capillary. Scale bar 5μm. Reproduced with permission from [4]
-
Figure 12. This electron tomography (thick section of about 300 nm) shows nanostructures connecting the TC and cardiomyocytes in adult mouse heart. The bridging structures (encircled) have 10-15 nm and suggest a molecular interaction between the Tp of one TC and the two adjacent cardiomyocytes. The dilated segment of Tp involved in the heterocellular connection (podom) - contains a mitochondrion (m).
-
Figure 13. High resolution light microscopy on toluidine blue stained semithin section (~1 µm thick ultramicrotome
-
Figure 14. Electron micrographs illustrates the relationships of TC (blue) with cardiomyocyte progenitors (CMP, brown). The Tp run parallel with the main axis of the CMP and seem to establish their direction of development.
-
Figure 15. Mice lung. Terminal bronchiole. At least 4 TC with their extensive Tp are visible between the epithelium and an arteriole (SMC - smooth muscle cells). Note, the striking labyrinthine network formed by Tp. In the upper part a mitosis (prophase) is obvious (orange circle). In addition, a putative stem cell (SC, green oval) is in close contacts with telocytes prolongations, establishing a heterocellular junctions, visible at higher magnification only). The tandem TC-SC forms, presumably, a TC-SC niche. In the lower part, a macrophage (MF) makes a stromal synapse with Tp.
-
Figure 16. Rat striated skeletal muscle (diaphargm). A typical TC (blue) with two convoluted Tp is shown, by transmission electron microscopy. Note, two shed vesicles (sv, violet). m-mitochondria, Ly-lymphocyte. The asterisks indicate, presumably, two empty exosomes, which probably released their vesicle content. BV-small blood vessel.
-
Figure 17. Rat jejunum. Toluidine blue stained Semithin Epon sections of jejunum mucosa showing the bottom of Lieberkuhn glands in transverse section and a telocyte (red star) surrounding one of the gland. Note the spindle-shape body sending off two telopodes, one of which measure at least 50 µm in the section plane.
-
Figure 18. Rat jejunum muscularis mucosa. The photo is a colour-enhanced digital micrograph of a black and white transmission electron microscopy image. A blue telopode of 14.2 µm in the section plane is illustrated around a nerve ending (green) between smooth muscle cells (brown).
-
Figure 19. Rat jejunum mucosa. A. This electron microscope image disclose a telopode (blue) in the profound region of lamina propria, close to the muscularis mucosa (brown) and in the proximity of a nerve ending (green). Note the alternating podom and podomere. B. Inset disclosing the organelle details of the podomere – intermediate filaments and free ribosomes, and of the podom – mitochondrion and endoplasmic reticulum cisternae. C. High resolution image illustrating in detail multiple mitochondria, endoplasmic reticulum cisterne and caveolae (arrow).
-
Figure 20. Rat jejunum. A. Photomicrograph of an interstitial cell of Cajal (violet) in muscularis externa. Note the large cell body which extend slender and relatively short connection towards the nerve endings (green). B. Digitally coloured TEM image showing a fibroblast (garnet) and a telocyte (blue) in the lamina propria. C. Coloured transmission electron micrograph (TEM) of a tangential section through a fibroblast cell. The internal structure can be seen, including the dilated rough endoplasmic reticulum (blue). responsible for synthesising collagen. In blue a telopode underlying the intestinal epithelium.
-
Figure 21. Rat jejunum mucosa. A telocyte (blue) telopode is engaged in different types of synapses with a plasma cell: two plain synapses (PS) and one multicontact synapse (MS) are seen.
-
Figure 22. Rat jejunum. A-E. 3-D image reconstruction from 5 serial sections of telocytes (blue) in lamina propria: telopodes branching in a 3-D pattern. Telocyte's nucleus is colored in violet. F-J. Computer-aided volume rendering and different-angle stereoscopic views of a telocyte (blue) surrounding a nerve fiber (green) in muscularis mucosa (dark red).
-
Figure 23. 3D reconstruction of a telocyte with its long telopodes.
-
Figure 24. A podom is a dilated portion of a telopode. Note the endoplasmic reticulum in yellow and mitochondria in red.
-
Figure 25. A color representation of convoluted telopodes (blue) and a shedding vesicle (magenta).
-
Figure 26. Shedding vesicles (magenta) emerged from the telopodes (blue) and are heading towards a stem cell (gray).
See also
- List of human cell types derived from the germ layers
- Interstitial cell of Cajal, a similar, and potentially equivalent, cell
References
- ^ Condrat, Carmen Elena; Barbu, Mădălina Gabriela; Thompson, Dana Claudia; Dănilă, Cezara Alina; Boboc, Andreea Elena; Suciu, Nicolae; Crețoiu, Dragoș; Voinea, Silviu Cristian (2021-01-01), Gorbunov, Nikolai V. (ed.), "Chapter One - Roles and distribution of telocytes in tissue organization in health and disease", Tissue Barriers in Disease, Injury and Regeneration, Elsevier, pp. 1–41, doi: 10.1016/b978-0-12-818561-2.00001-1, ISBN 978-0-12-818561-2, S2CID 237997655
- ^ a b Popescu, L. M.; Faussone-Pellegrini, Maria-Simonetta (April 2010). "TELOCYTES - a case of serendipity: the winding way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-Like Cells (ICLC) to TELOCYTES". Journal of Cellular and Molecular Medicine. 14 (4): 729–740. doi: 10.1111/j.1582-4934.2010.01059.x. ISSN 1582-4934. PMC 3823108. PMID 20367664.
- ^ a b Suciu, Laura; Popescu, Laurenţiu M.; Gherghiceanu, Mihaela; Regalia, Teodor; Nicolescu, Mihnea I.; Hinescu, Mihail E.; Faussone-Pellegrini, Maria-Simonetta (2010). "Telocytes in human term placenta: morphology and phenotype". Cells Tissues Organs. 192 (5): 325–339. doi: 10.1159/000319467. ISSN 1422-6421. PMID 20664249. S2CID 27675409.
- ^ Popescu, L M; Manole, C G; Gherghiceanu, M; Ardelean, A; Nicolescu, M I; Hinescu, M E; Kostin, S (August 2010). "Telocytes in human epicardium". Journal of Cellular and Molecular Medicine. 14 (8): 2085–2093. doi: 10.1111/j.1582-4934.2010.01129.x. ISSN 1582-1838. PMC 3823000. PMID 20629996.
- ^ Gherghiceanu, Mihaela; Manole, C. G.; Popescu, L. M. (September 2010). "Telocytes in endocardium: electron microscope evidence". Journal of Cellular and Molecular Medicine. 14 (9): 2330–2334. doi: 10.1111/j.1582-4934.2010.01133.x. ISSN 1582-4934. PMC 3822573. PMID 20716125.
- ^ Popescu, LM; Gherghiceanu, M; Kostin, S (2011). "Telocytes and heart renewing". In Wang, P; Kuo, CH; Takeda, N; Singal, PK (eds.). Adaptation biology and medicine, vol 6. Cell adaptations and challenges. Vol. 6. New Delhi: Narosa Publishing. pp. 17–39.
- ^ Gherghiceanu, Mihaela; Popescu, L M (April 2010). "Cardiomyocyte precursors and telocytes in epicardial stem cell niche: electron microscope images". Journal of Cellular and Molecular Medicine. 14 (4): 871–877. doi: 10.1111/j.1582-4934.2010.01060.x. ISSN 1582-1838. PMC 3823118. PMID 20367663.
- ^ a b Hinescu, Mihail E.; Gherghiceanu, Mihaela; Suciu, Laura; Popescu, Laurentiu M. (February 2011). "Telocytes in pleura: two- and three-dimensional imaging by transmission electron microscopy". Cell and Tissue Research. 343 (2): 389–397. doi: 10.1007/s00441-010-1095-0. ISSN 0302-766X. PMC 3032227. PMID 21174125.
- ^ Bani, Daniele; Formigli, Lucia; Gherghiceanu, Mihaela; Faussone-Pellegrini, Maria-Simonetta (October 2010). "Telocytes as supporting cells for myocardial tissue organization in developing and adult heart". Journal of Cellular and Molecular Medicine. 14 (10): 2531–2538. doi: 10.1111/j.1582-4934.2010.01119.x. ISSN 1582-4934. PMC 3823169. PMID 20977627.
- ^ Kostin, Sawa (July 2010). "Myocardial telocytes: a specific new cellular entity". Journal of Cellular and Molecular Medicine. 14 (7): 1917–1921. doi: 10.1111/j.1582-4934.2010.01111.x. ISSN 1582-1838. PMC 3823273. PMID 20604817.
- ^ Groot, Adriana C Gittenberger-de; Winter, Elisabeth M; Poelmann, Robert E (May 2010). "Epicardium-derived cells (EPDCs) in development, cardiac disease and repair of ischemia". Journal of Cellular and Molecular Medicine. 14 (5): 1056–1060. doi: 10.1111/j.1582-4934.2010.01077.x. ISSN 1582-1838. PMC 3822740. PMID 20646126.
- ^ D, Klumpp; Re, Horch; U, Kneser; Jp, Beier (November 2010). "Engineering skeletal muscle tissue--new perspectives in vitro and in vivo". Journal of Cellular and Molecular Medicine. 14 (11): 2622–2629. doi: 10.1111/j.1582-4934.2010.01183.x. PMC 4373482. PMID 21091904.
- ^ Tommila M, Granulation tissue formation. The effect of hydroxyapatite coating of cellulose on cellular differentiation. PhD Thesis, University of Turku, Finland.
- ^ Zhou, Jin; Zhang, Ye; Wen, Xinyu; Cao, Junkai; Li, Dexue; Lin, Qiuxia; Wang, Haibin; Liu, Zhiqiang; Duan, Cuimi; Wu, Kuiwu; Wang, Changyong (November 2010). "Telocytes accompanying cardiomyocyte in primary culture: two- and three-dimensional culture environment". Journal of Cellular and Molecular Medicine. 14 (11): 2641–2645. doi: 10.1111/j.1582-4934.2010.01186.x. ISSN 1582-1838. PMC 4373485. PMID 21158014.
- ^ Limana, Federica; Capogrossi, Maurizio C.; Germani, Antonia (January 2011). "The epicardium in cardiac repair: from the stem cell view". Pharmacology & Therapeutics. 129 (1): 82–96. doi: 10.1016/j.pharmthera.2010.09.002. ISSN 1879-016X. PMID 20937304.
- ^ Carmona, I. Cantarero; Bartolomé, M. J. Luesma; Escribano, C. Junquera (January 2011). "Identification of telocytes in the lamina propria of rat duodenum: transmission electron microscopy". Journal of Cellular and Molecular Medicine. 15 (1): 26–30. doi: 10.1111/j.1582-4934.2010.01207.x. PMC 3822490. PMID 21054782.
- ^ Kostin, Sawa (April 2011). "Types of Cardiomyocyte Death and Clinical Outcomes in Patients With Heart Failure". Journal of the American College of Cardiology. 57 (14): 1532–1534. doi: 10.1016/j.jacc.2010.10.049. PMID 21453831.
- ^ Radenkovic, Goran (January 2012). "Two patterns of development of interstitial cells of Cajal in the human duodenum". Journal of Cellular and Molecular Medicine. 16 (1): 185–192. doi: 10.1111/j.1582-4934.2011.01287.x. PMC 3823104. PMID 21352475.
- ^ Russell, Jamie L.; Goetsch, Sean C.; Gaiano, Nicholas R.; Hill, Joseph A.; Olson, Eric N.; Schneider, Jay W. (2011-01-07). "A dynamic notch injury response activates epicardium and contributes to fibrosis repair". Circulation Research. 108 (1): 51–59. doi: 10.1161/CIRCRESAHA.110.233262. ISSN 1524-4571. PMC 3042596. PMID 21106942.
- ^ Creţoiu, Sanda M.; Creţoiu, Dragos; Popescu, Laurentiu M. (November 2012). "Human myometrium - the ultrastructural 3D network of telocytes". Journal of Cellular and Molecular Medicine. 16 (11): 2844–2849. doi: 10.1111/j.1582-4934.2012.01651.x. PMC 4118253. PMID 23009098.
- ^ Cretoiu, Sanda M; Cretoiu, Dragos; Marin, Adela; Radu, Beatrice Mihaela; Popescu, Laurentiu M (April 2013). "Telocytes: ultrastructural, immunohistochemical and electrophysiological characteristics in human myometrium". Reproduction. 145 (4): 357–370. doi: 10.1530/REP-12-0369. ISSN 1470-1626. PMC 3636525. PMID 23404846.
- ^ Zheng, Y.; Li, H.; Manole, C. G.; Sun, A.; Ge, J.; Wang, X. (October 2011). "Telocytes in trachea and lungs". Journal of Cellular and Molecular Medicine. 15 (10): 2262–2268. doi: 10.1111/j.1582-4934.2011.01404.x. PMC 4394233. PMID 21810171.
- ^ a b Zheng, Yonghua; Cretoiu, Dragos; Yan, Guoquan; Cretoiu, Sanda Maria; Popescu, Laurentiu M.; Wang, Xiangdong (April 2014). "Comparative proteomic analysis of human lung telocytes with fibroblasts". Journal of Cellular and Molecular Medicine. 18 (4): 568–589. doi: 10.1111/jcmm.12290. PMC 4000110. PMID 24674459.
- ^ Nicolescu, Mihnea I.; Popescu, Laurentiu M. (August 2012). "Telocytes in the Interstitium of Human Exocrine Pancreas: Ultrastructural Evidence". Pancreas. 41 (6): 949–956. doi: 10.1097/MPA.0b013e31823fbded. ISSN 0885-3177. PMID 22318257. S2CID 23643116.
- ^ Li, Liping; Lin, Miao; Li, Long; Wang, Rulin; Zhang, Chao; Qi, Guisheng; Xu, Ming; Rong, Ruiming; Zhu, Tongyu (June 2014). "Renal telocytes contribute to the repair of ischemically injured renal tubules". Journal of Cellular and Molecular Medicine. 18 (6): 1144–1156. doi: 10.1111/jcmm.12274. PMC 4508154. PMID 24758589.
- ^ Qi, Guisheng; Lin, Miao; Xu, Ming; Manole, C. G.; Wang, Xiangdong; Zhu, Tongyu (December 2012). "Telocytes in the human kidney cortex". Journal of Cellular and Molecular Medicine. 16 (12): 3116–3122. doi: 10.1111/j.1582-4934.2012.01582.x. PMC 4393739. PMID 23241355.
- ^ Mandache, E.; Gherghiceanu, M.; Macarie, C.; Kostin, S.; Popescu, L. M. (December 2010). "Telocytes in human isolated atrial amyloidosis: ultrastructural remodelling". Journal of Cellular and Molecular Medicine. 14 (12): 2739–2747. doi: 10.1111/j.1582-4934.2010.01200.x. ISSN 1582-4934. PMC 3822724. PMID 21040457.
- ^ Polykandriotis, E.; Popescu, L. M.; Horch, R. E. (October 2010). "Regenerative medicine: then and now--an update of recent history into future possibilities". Journal of Cellular and Molecular Medicine. 14 (10): 2350–2358. doi: 10.1111/j.1582-4934.2010.01169.x. ISSN 1582-4934. PMC 3823153. PMID 20825521.
- ^ Zhao, Baoyin; Liao, Zhaofu; Chen, Shang; Yuan, Ziqiang; Yilin, Chen; Lee, Kenneth K.H.; Qi, Xufeng; Shen, Xiaotao; Zheng, Xin; Quinn, Thomas; Cai, Dongqing (May 2014). "Intramyocardial transplantation of cardiac telocytes decreases myocardial infarction and improves post-infarcted cardiac function in rats". Journal of Cellular and Molecular Medicine. 18 (5): 780–789. doi: 10.1111/jcmm.12259. PMC 4119384. PMID 24655344.
This article has multiple issues. Please help
improve it or discuss these issues on the
talk page. (
Learn how and when to remove these template messages)
|
Telocytes are a type of interstitial ( stromal) cells with very long (tens to hundreds of micrometres) and very thin prolongations (mostly below the resolving power of light microscopy) called telopodes. [1]








Rationale for the term telocyte
Professor Laurențiu M. Popescu's group from Bucharest, Romania described a new type of cell. Popescu coined the terms telocytes (TC) for these cells, and telopodes (Tp) [2] for their extremely long but thin prolongations [2] [3] [4] [5] [6] [7] [8] in order to prevent further confusion with other interstitial (stromal) cells (e.g., fibroblast, fibroblast-like cells, myofibroblast, mesenchymal cells). Telopodes present an alternation of thin segments, podomeres (with caliber mostly under 200 nm, below the resolving power of light microscopy) and dilated segments, podoms, which accommodate mitochondria, (rough) endoplasmic reticulum and caveolae - the so-called "Ca2+ uptake/release units". The concept of TC was promptly adopted by other laboratories, as well. [9] [10] [11] [12] [13] [14] [15] [16] [17] [18] [19]
Telocytes and/or fibroblasts?
The interstitium ( stroma) is in most of the cases seen as a connecting "device" for the specific structures of an organ. Usually, people perceive interstitial cells as being mainly (or even, only) fibroblasts. However, fibroblasts have the function of generating connective tissue matrix, specifically, collagen. The distinction between TC and fibroblasts is obvious since they have different ultrastructure and phenotype. Therefore, their functions should be mostly different: TC - intercellular signaling (connections), but fibroblasts - collagen synthesis. In other words, TC are "more" functionally oriented while fibroblasts are "more" structurally oriented, responsible for fibrosis.
There are some clear ultrastructural features that differentiate telocytes from fibroblasts. For instance, the general aspect of TC is of a small oval ( piriform/spindle/triangular/ stellate)-shaped cellular body, containing a nucleus surrounded by a small amount of cytoplasm. Anyway, the shape of the cell body depends on the number of Tp. TC cellular body average dimensions are, as measured on EM images, 9.3 μm ± 3.2 μm (min. 6.3μm; max. 16.4 μm). Fibroblast nucleus is typically euchromatic, but TC nucleus is mostly heterochromatic. Mitochondria represent only 2% of cell body volume and the Golgi complex is small in TC. Fibroblasts Golgi complex is prominent and the rough endoplasmic reticulum is very well developed (usually 5-12%) of cell volume.
Since telopodes are distinctive for telocytes, here are their main features:
- Number: 1–5 (frequently only 2–3 telopodes are observed on a single section, depending on site and angle of section, since their 3D convolutions prevent them to be observed at their full length in a 2D very thin section);
- Length: tens – up to hundreds of μm, as measured on EM images (e.g. Figs. 2-10). However, under favorable conditions in cell cultures, their entire length can be captured in several successive images (Fig. 1);
- Thickness: uneven caliber, mostly below 0.2 μm (below the resolving power of light microscopy), visible under electron microscopy;
- Moniliform aspect: podoms and podomeres; average caliber of podomeres: 0.1 μm ± 0.05μm, min. = 0.003 μm; max. = 0.24 μm; Podoms accommodate: mitochondria, (rough) endoplasmic reticulum, caveolae, a trio called ‘Ca2+-uptake/release units’.
- Branching, with a dichotomous pattern;
- Organization in a labyrinthine system, forming a 3D network anchored by hetero- and homocellular junctions.
Summary
Here is shown visual evidence (electron microscopy, electron tomography, phase contrast microscopy) for the existence of Telocytes (TC) in many organs from human and rodents. TC and Tp, and also podoms and podomeres were found in:
- cavitary organs:
- heart ( endo-, myo-, and pericardium);
- stomach and intestine, with mesentery;
- gall bladder;
- uterus and fallopian tube; [20] [21]
- non-cavitary organs:
- lungs and pleura; [8] [22] [23]
- pancreas ( exocrine gland); [24]
- mammary gland;
- placenta; [3]
- kidneys; [25] [26]
Recent evidence shows the involvement of TC in pathology. [27] TC are strategically located in between blood vessels ( capillaries), nerve endings and the specific resident cell populations of a given organ. TC establish via Tp homo- and heterocellular junctions and release shed vesicles and exosomes.
Perspectives: regenerative medicine
TC and SC make a tandem (due to specific intercellular junctions) within the so-called SC niches, at least in heart [28] and lungs. Hence, TC could be key-players in regenerating and repair of some organs. The tandem TC-SC could be a better option for therapy rather than SC alone. Published studies suggest that cardiac TCs could be regarded as a potential cell source for therapeutic use to improve cardiac repair and function after a myocardial infarction, either alone or in tandem with SC. [29] Recent data show that TCs are completely different from FBs, using a quantitative proteomics approach, suggesting that TCs might play specific roles in mechanical sensing and mechanochemical conversion task, tissue homoeostasis and remodelling/renewal. [23]
Figures
-
Figure 9. Human mammary gland stroma: TEM; original magnification 9,100x. A: Lymphocyte establishing a multicontact synapse (MS) with a TC. The blue rectangle shows the synaptic ‘kiss and run’ region. The synaptic membranes appear traced in B (violet - TC, orange - lymphocyte). The distances between membranes are shown in C. Note (asterisk) a peculiar conformation of ER connecting mitochondria with the cell surface, suggestive for a possible role in synaptic Ca2+ homeostasis. Reproduced with permission from [22]
-
Figure 10. Scanning electron micrograph of monkey left ventricular myocardium. A typical TC is located across the cardiomyocytes, in close contacts with blood capillaries. Note, the cardiomyocytes striations and the openings of T tubules.
-
Figure 11. Digitally coloured electron micrograph of mouse ventricular endocardium (burgundy). TC (blue) make an interstitial network in the heart. Subendocardial telocytes (TC1) sends Tp between cardiomyocytes (CM) and communicate with TC2. Cap, blood capillary. Scale bar 5μm. Reproduced with permission from [4]
-
Figure 12. This electron tomography (thick section of about 300 nm) shows nanostructures connecting the TC and cardiomyocytes in adult mouse heart. The bridging structures (encircled) have 10-15 nm and suggest a molecular interaction between the Tp of one TC and the two adjacent cardiomyocytes. The dilated segment of Tp involved in the heterocellular connection (podom) - contains a mitochondrion (m).
-
Figure 13. High resolution light microscopy on toluidine blue stained semithin section (~1 µm thick ultramicrotome
-
Figure 14. Electron micrographs illustrates the relationships of TC (blue) with cardiomyocyte progenitors (CMP, brown). The Tp run parallel with the main axis of the CMP and seem to establish their direction of development.
-
Figure 15. Mice lung. Terminal bronchiole. At least 4 TC with their extensive Tp are visible between the epithelium and an arteriole (SMC - smooth muscle cells). Note, the striking labyrinthine network formed by Tp. In the upper part a mitosis (prophase) is obvious (orange circle). In addition, a putative stem cell (SC, green oval) is in close contacts with telocytes prolongations, establishing a heterocellular junctions, visible at higher magnification only). The tandem TC-SC forms, presumably, a TC-SC niche. In the lower part, a macrophage (MF) makes a stromal synapse with Tp.
-
Figure 16. Rat striated skeletal muscle (diaphargm). A typical TC (blue) with two convoluted Tp is shown, by transmission electron microscopy. Note, two shed vesicles (sv, violet). m-mitochondria, Ly-lymphocyte. The asterisks indicate, presumably, two empty exosomes, which probably released their vesicle content. BV-small blood vessel.
-
Figure 17. Rat jejunum. Toluidine blue stained Semithin Epon sections of jejunum mucosa showing the bottom of Lieberkuhn glands in transverse section and a telocyte (red star) surrounding one of the gland. Note the spindle-shape body sending off two telopodes, one of which measure at least 50 µm in the section plane.
-
Figure 18. Rat jejunum muscularis mucosa. The photo is a colour-enhanced digital micrograph of a black and white transmission electron microscopy image. A blue telopode of 14.2 µm in the section plane is illustrated around a nerve ending (green) between smooth muscle cells (brown).
-
Figure 19. Rat jejunum mucosa. A. This electron microscope image disclose a telopode (blue) in the profound region of lamina propria, close to the muscularis mucosa (brown) and in the proximity of a nerve ending (green). Note the alternating podom and podomere. B. Inset disclosing the organelle details of the podomere – intermediate filaments and free ribosomes, and of the podom – mitochondrion and endoplasmic reticulum cisternae. C. High resolution image illustrating in detail multiple mitochondria, endoplasmic reticulum cisterne and caveolae (arrow).
-
Figure 20. Rat jejunum. A. Photomicrograph of an interstitial cell of Cajal (violet) in muscularis externa. Note the large cell body which extend slender and relatively short connection towards the nerve endings (green). B. Digitally coloured TEM image showing a fibroblast (garnet) and a telocyte (blue) in the lamina propria. C. Coloured transmission electron micrograph (TEM) of a tangential section through a fibroblast cell. The internal structure can be seen, including the dilated rough endoplasmic reticulum (blue). responsible for synthesising collagen. In blue a telopode underlying the intestinal epithelium.
-
Figure 21. Rat jejunum mucosa. A telocyte (blue) telopode is engaged in different types of synapses with a plasma cell: two plain synapses (PS) and one multicontact synapse (MS) are seen.
-
Figure 22. Rat jejunum. A-E. 3-D image reconstruction from 5 serial sections of telocytes (blue) in lamina propria: telopodes branching in a 3-D pattern. Telocyte's nucleus is colored in violet. F-J. Computer-aided volume rendering and different-angle stereoscopic views of a telocyte (blue) surrounding a nerve fiber (green) in muscularis mucosa (dark red).
-
Figure 23. 3D reconstruction of a telocyte with its long telopodes.
-
Figure 24. A podom is a dilated portion of a telopode. Note the endoplasmic reticulum in yellow and mitochondria in red.
-
Figure 25. A color representation of convoluted telopodes (blue) and a shedding vesicle (magenta).
-
Figure 26. Shedding vesicles (magenta) emerged from the telopodes (blue) and are heading towards a stem cell (gray).
See also
- List of human cell types derived from the germ layers
- Interstitial cell of Cajal, a similar, and potentially equivalent, cell
References
- ^ Condrat, Carmen Elena; Barbu, Mădălina Gabriela; Thompson, Dana Claudia; Dănilă, Cezara Alina; Boboc, Andreea Elena; Suciu, Nicolae; Crețoiu, Dragoș; Voinea, Silviu Cristian (2021-01-01), Gorbunov, Nikolai V. (ed.), "Chapter One - Roles and distribution of telocytes in tissue organization in health and disease", Tissue Barriers in Disease, Injury and Regeneration, Elsevier, pp. 1–41, doi: 10.1016/b978-0-12-818561-2.00001-1, ISBN 978-0-12-818561-2, S2CID 237997655
- ^ a b Popescu, L. M.; Faussone-Pellegrini, Maria-Simonetta (April 2010). "TELOCYTES - a case of serendipity: the winding way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-Like Cells (ICLC) to TELOCYTES". Journal of Cellular and Molecular Medicine. 14 (4): 729–740. doi: 10.1111/j.1582-4934.2010.01059.x. ISSN 1582-4934. PMC 3823108. PMID 20367664.
- ^ a b Suciu, Laura; Popescu, Laurenţiu M.; Gherghiceanu, Mihaela; Regalia, Teodor; Nicolescu, Mihnea I.; Hinescu, Mihail E.; Faussone-Pellegrini, Maria-Simonetta (2010). "Telocytes in human term placenta: morphology and phenotype". Cells Tissues Organs. 192 (5): 325–339. doi: 10.1159/000319467. ISSN 1422-6421. PMID 20664249. S2CID 27675409.
- ^ Popescu, L M; Manole, C G; Gherghiceanu, M; Ardelean, A; Nicolescu, M I; Hinescu, M E; Kostin, S (August 2010). "Telocytes in human epicardium". Journal of Cellular and Molecular Medicine. 14 (8): 2085–2093. doi: 10.1111/j.1582-4934.2010.01129.x. ISSN 1582-1838. PMC 3823000. PMID 20629996.
- ^ Gherghiceanu, Mihaela; Manole, C. G.; Popescu, L. M. (September 2010). "Telocytes in endocardium: electron microscope evidence". Journal of Cellular and Molecular Medicine. 14 (9): 2330–2334. doi: 10.1111/j.1582-4934.2010.01133.x. ISSN 1582-4934. PMC 3822573. PMID 20716125.
- ^ Popescu, LM; Gherghiceanu, M; Kostin, S (2011). "Telocytes and heart renewing". In Wang, P; Kuo, CH; Takeda, N; Singal, PK (eds.). Adaptation biology and medicine, vol 6. Cell adaptations and challenges. Vol. 6. New Delhi: Narosa Publishing. pp. 17–39.
- ^ Gherghiceanu, Mihaela; Popescu, L M (April 2010). "Cardiomyocyte precursors and telocytes in epicardial stem cell niche: electron microscope images". Journal of Cellular and Molecular Medicine. 14 (4): 871–877. doi: 10.1111/j.1582-4934.2010.01060.x. ISSN 1582-1838. PMC 3823118. PMID 20367663.
- ^ a b Hinescu, Mihail E.; Gherghiceanu, Mihaela; Suciu, Laura; Popescu, Laurentiu M. (February 2011). "Telocytes in pleura: two- and three-dimensional imaging by transmission electron microscopy". Cell and Tissue Research. 343 (2): 389–397. doi: 10.1007/s00441-010-1095-0. ISSN 0302-766X. PMC 3032227. PMID 21174125.
- ^ Bani, Daniele; Formigli, Lucia; Gherghiceanu, Mihaela; Faussone-Pellegrini, Maria-Simonetta (October 2010). "Telocytes as supporting cells for myocardial tissue organization in developing and adult heart". Journal of Cellular and Molecular Medicine. 14 (10): 2531–2538. doi: 10.1111/j.1582-4934.2010.01119.x. ISSN 1582-4934. PMC 3823169. PMID 20977627.
- ^ Kostin, Sawa (July 2010). "Myocardial telocytes: a specific new cellular entity". Journal of Cellular and Molecular Medicine. 14 (7): 1917–1921. doi: 10.1111/j.1582-4934.2010.01111.x. ISSN 1582-1838. PMC 3823273. PMID 20604817.
- ^ Groot, Adriana C Gittenberger-de; Winter, Elisabeth M; Poelmann, Robert E (May 2010). "Epicardium-derived cells (EPDCs) in development, cardiac disease and repair of ischemia". Journal of Cellular and Molecular Medicine. 14 (5): 1056–1060. doi: 10.1111/j.1582-4934.2010.01077.x. ISSN 1582-1838. PMC 3822740. PMID 20646126.
- ^ D, Klumpp; Re, Horch; U, Kneser; Jp, Beier (November 2010). "Engineering skeletal muscle tissue--new perspectives in vitro and in vivo". Journal of Cellular and Molecular Medicine. 14 (11): 2622–2629. doi: 10.1111/j.1582-4934.2010.01183.x. PMC 4373482. PMID 21091904.
- ^ Tommila M, Granulation tissue formation. The effect of hydroxyapatite coating of cellulose on cellular differentiation. PhD Thesis, University of Turku, Finland.
- ^ Zhou, Jin; Zhang, Ye; Wen, Xinyu; Cao, Junkai; Li, Dexue; Lin, Qiuxia; Wang, Haibin; Liu, Zhiqiang; Duan, Cuimi; Wu, Kuiwu; Wang, Changyong (November 2010). "Telocytes accompanying cardiomyocyte in primary culture: two- and three-dimensional culture environment". Journal of Cellular and Molecular Medicine. 14 (11): 2641–2645. doi: 10.1111/j.1582-4934.2010.01186.x. ISSN 1582-1838. PMC 4373485. PMID 21158014.
- ^ Limana, Federica; Capogrossi, Maurizio C.; Germani, Antonia (January 2011). "The epicardium in cardiac repair: from the stem cell view". Pharmacology & Therapeutics. 129 (1): 82–96. doi: 10.1016/j.pharmthera.2010.09.002. ISSN 1879-016X. PMID 20937304.
- ^ Carmona, I. Cantarero; Bartolomé, M. J. Luesma; Escribano, C. Junquera (January 2011). "Identification of telocytes in the lamina propria of rat duodenum: transmission electron microscopy". Journal of Cellular and Molecular Medicine. 15 (1): 26–30. doi: 10.1111/j.1582-4934.2010.01207.x. PMC 3822490. PMID 21054782.
- ^ Kostin, Sawa (April 2011). "Types of Cardiomyocyte Death and Clinical Outcomes in Patients With Heart Failure". Journal of the American College of Cardiology. 57 (14): 1532–1534. doi: 10.1016/j.jacc.2010.10.049. PMID 21453831.
- ^ Radenkovic, Goran (January 2012). "Two patterns of development of interstitial cells of Cajal in the human duodenum". Journal of Cellular and Molecular Medicine. 16 (1): 185–192. doi: 10.1111/j.1582-4934.2011.01287.x. PMC 3823104. PMID 21352475.
- ^ Russell, Jamie L.; Goetsch, Sean C.; Gaiano, Nicholas R.; Hill, Joseph A.; Olson, Eric N.; Schneider, Jay W. (2011-01-07). "A dynamic notch injury response activates epicardium and contributes to fibrosis repair". Circulation Research. 108 (1): 51–59. doi: 10.1161/CIRCRESAHA.110.233262. ISSN 1524-4571. PMC 3042596. PMID 21106942.
- ^ Creţoiu, Sanda M.; Creţoiu, Dragos; Popescu, Laurentiu M. (November 2012). "Human myometrium - the ultrastructural 3D network of telocytes". Journal of Cellular and Molecular Medicine. 16 (11): 2844–2849. doi: 10.1111/j.1582-4934.2012.01651.x. PMC 4118253. PMID 23009098.
- ^ Cretoiu, Sanda M; Cretoiu, Dragos; Marin, Adela; Radu, Beatrice Mihaela; Popescu, Laurentiu M (April 2013). "Telocytes: ultrastructural, immunohistochemical and electrophysiological characteristics in human myometrium". Reproduction. 145 (4): 357–370. doi: 10.1530/REP-12-0369. ISSN 1470-1626. PMC 3636525. PMID 23404846.
- ^ Zheng, Y.; Li, H.; Manole, C. G.; Sun, A.; Ge, J.; Wang, X. (October 2011). "Telocytes in trachea and lungs". Journal of Cellular and Molecular Medicine. 15 (10): 2262–2268. doi: 10.1111/j.1582-4934.2011.01404.x. PMC 4394233. PMID 21810171.
- ^ a b Zheng, Yonghua; Cretoiu, Dragos; Yan, Guoquan; Cretoiu, Sanda Maria; Popescu, Laurentiu M.; Wang, Xiangdong (April 2014). "Comparative proteomic analysis of human lung telocytes with fibroblasts". Journal of Cellular and Molecular Medicine. 18 (4): 568–589. doi: 10.1111/jcmm.12290. PMC 4000110. PMID 24674459.
- ^ Nicolescu, Mihnea I.; Popescu, Laurentiu M. (August 2012). "Telocytes in the Interstitium of Human Exocrine Pancreas: Ultrastructural Evidence". Pancreas. 41 (6): 949–956. doi: 10.1097/MPA.0b013e31823fbded. ISSN 0885-3177. PMID 22318257. S2CID 23643116.
- ^ Li, Liping; Lin, Miao; Li, Long; Wang, Rulin; Zhang, Chao; Qi, Guisheng; Xu, Ming; Rong, Ruiming; Zhu, Tongyu (June 2014). "Renal telocytes contribute to the repair of ischemically injured renal tubules". Journal of Cellular and Molecular Medicine. 18 (6): 1144–1156. doi: 10.1111/jcmm.12274. PMC 4508154. PMID 24758589.
- ^ Qi, Guisheng; Lin, Miao; Xu, Ming; Manole, C. G.; Wang, Xiangdong; Zhu, Tongyu (December 2012). "Telocytes in the human kidney cortex". Journal of Cellular and Molecular Medicine. 16 (12): 3116–3122. doi: 10.1111/j.1582-4934.2012.01582.x. PMC 4393739. PMID 23241355.
- ^ Mandache, E.; Gherghiceanu, M.; Macarie, C.; Kostin, S.; Popescu, L. M. (December 2010). "Telocytes in human isolated atrial amyloidosis: ultrastructural remodelling". Journal of Cellular and Molecular Medicine. 14 (12): 2739–2747. doi: 10.1111/j.1582-4934.2010.01200.x. ISSN 1582-4934. PMC 3822724. PMID 21040457.
- ^ Polykandriotis, E.; Popescu, L. M.; Horch, R. E. (October 2010). "Regenerative medicine: then and now--an update of recent history into future possibilities". Journal of Cellular and Molecular Medicine. 14 (10): 2350–2358. doi: 10.1111/j.1582-4934.2010.01169.x. ISSN 1582-4934. PMC 3823153. PMID 20825521.
- ^ Zhao, Baoyin; Liao, Zhaofu; Chen, Shang; Yuan, Ziqiang; Yilin, Chen; Lee, Kenneth K.H.; Qi, Xufeng; Shen, Xiaotao; Zheng, Xin; Quinn, Thomas; Cai, Dongqing (May 2014). "Intramyocardial transplantation of cardiac telocytes decreases myocardial infarction and improves post-infarcted cardiac function in rats". Journal of Cellular and Molecular Medicine. 18 (5): 780–789. doi: 10.1111/jcmm.12259. PMC 4119384. PMID 24655344.
![Figure 9. Human mammary gland stroma: TEM; original magnification 9,100x. A: Lymphocyte establishing a multicontact synapse (MS) with a TC. The blue rectangle shows the synaptic ‘kiss and run’ region. The synaptic membranes appear traced in B (violet - TC, orange - lymphocyte). The distances between membranes are shown in C. Note (asterisk) a peculiar conformation of ER connecting mitochondria with the cell surface, suggestive for a possible role in synaptic Ca2+ homeostasis. Reproduced with permission from [22]](https://upload.wikimedia.org/wikipedia/commons/thumb/2/20/Telocytes-Fig_9.tif/lossy-page1-82px-Telocytes-Fig_9.tif.jpg)
![Figure 11. Digitally coloured electron micrograph of mouse ventricular endocardium (burgundy). TC (blue) make an interstitial network in the heart. Subendocardial telocytes (TC1) sends Tp between cardiomyocytes (CM) and communicate with TC2. Cap, blood capillary. Scale bar 5μm. Reproduced with permission from [4]](https://upload.wikimedia.org/wikipedia/commons/thumb/e/e8/Telocytes-Fig_11.tif/lossy-page1-120px-Telocytes-Fig_11.tif.jpg)