 | |
 | |
| Clinical data | |
|---|---|
|
Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 5–8 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard ( EPA) | |
| Chemical and physical data | |
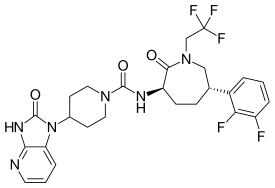
| Formula | C26H27F5N6O3 |
| Molar mass | 566.533 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
| | |
Telcagepant ( INN) (code name MK-0974) is a calcitonin gene-related peptide receptor antagonist which was an investigational drug for the acute treatment and prevention of migraine, developed by Merck & Co. [1]
In the acute treatment of migraine, it was found to have equal efficacy to rizatriptan [2] and zolmitriptan. [3]
A Phase IIa clinical trial studying telcagepant for the prophylaxis of episodic migraine was stopped on March 26, 2009, after the "identification of two patients with significant elevations in serum transaminases". [4] A memo to study locations stated that telcagepant had preliminarily been reported to increase the hepatic liver enzyme alanine transaminase (ALT) levels in "11 out of 660 randomized (double-blinded) study participants." All study participants were told to stop taking the medication. [5]
In July 2011, Merck announced that it had discontinued development of telcagepant. [6]
- ^ Molecule of the Month February 2009
- ^ Ho TW, Mannix LK, Fan X, Assaid C, Furtek C, Jones CJ, et al. (April 2008). "Randomized controlled trial of an oral CGRP receptor antagonist, MK-0974, in acute treatment of migraine". Neurology. 70 (16): 1304–12. doi: 10.1212/01.WNL.0000286940.29755.61. PMID 17914062. S2CID 11612471.
- ^ Ho TW, Ferrari MD, Dodick DW, Galet V, Kost J, Fan X, et al. (December 2008). "Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmitriptan for acute migraine: a randomised, placebo-controlled, parallel-treatment trial". Lancet. 372 (9656): 2115–23. doi: 10.1016/S0140-6736(08)61626-8. PMID 19036425. S2CID 43069027.
- ^ Clinical trial number NCT00797667 for "MK0974 for Migraine Prophylaxis in Patients With Episodic Migraine" at ClinicalTrials.gov
- ^ Merck & Co.: Memo to all US study locations involved in protocol MK0974-049
- ^ "Press release: Merck Announces Second Quarter 2011 Financial Results". Merck. July 29, 2011. Archived from the original on April 12, 2013.
 | |
 | |
| Clinical data | |
|---|---|
|
Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 5–8 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard ( EPA) | |
| Chemical and physical data | |
| Formula | C26H27F5N6O3 |
| Molar mass | 566.533 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
| | |
Telcagepant ( INN) (code name MK-0974) is a calcitonin gene-related peptide receptor antagonist which was an investigational drug for the acute treatment and prevention of migraine, developed by Merck & Co. [1]
In the acute treatment of migraine, it was found to have equal efficacy to rizatriptan [2] and zolmitriptan. [3]
A Phase IIa clinical trial studying telcagepant for the prophylaxis of episodic migraine was stopped on March 26, 2009, after the "identification of two patients with significant elevations in serum transaminases". [4] A memo to study locations stated that telcagepant had preliminarily been reported to increase the hepatic liver enzyme alanine transaminase (ALT) levels in "11 out of 660 randomized (double-blinded) study participants." All study participants were told to stop taking the medication. [5]
In July 2011, Merck announced that it had discontinued development of telcagepant. [6]
- ^ Molecule of the Month February 2009
- ^ Ho TW, Mannix LK, Fan X, Assaid C, Furtek C, Jones CJ, et al. (April 2008). "Randomized controlled trial of an oral CGRP receptor antagonist, MK-0974, in acute treatment of migraine". Neurology. 70 (16): 1304–12. doi: 10.1212/01.WNL.0000286940.29755.61. PMID 17914062. S2CID 11612471.
- ^ Ho TW, Ferrari MD, Dodick DW, Galet V, Kost J, Fan X, et al. (December 2008). "Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmitriptan for acute migraine: a randomised, placebo-controlled, parallel-treatment trial". Lancet. 372 (9656): 2115–23. doi: 10.1016/S0140-6736(08)61626-8. PMID 19036425. S2CID 43069027.
- ^ Clinical trial number NCT00797667 for "MK0974 for Migraine Prophylaxis in Patients With Episodic Migraine" at ClinicalTrials.gov
- ^ Merck & Co.: Memo to all US study locations involved in protocol MK0974-049
- ^ "Press release: Merck Announces Second Quarter 2011 Financial Results". Merck. July 29, 2011. Archived from the original on April 12, 2013.