
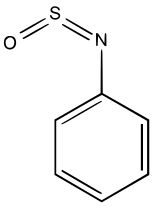
Sulfinylamines (formerly N-sulfinyl amines) are organosulfur compounds with the formula RNSO where R = an organic substituent. These compounds are, formally speaking, derivatives of HN=S=O, i.e. analogues of sulfur dioxide and of sulfur diimide. A common example is N-sulfinylaniline. Sulfinyl amines are dienophile. [1] They undergo [2+2] cycloaddition to ketenes. [2]
According to X-ray crystallography, sulfinylamines have planar C-N=S=O cores with syn geometry. [3]
Sulfinylamines can be made when thionyl chloride SOCl2 reacts with a primary amine. [4]
Mixtures of phosphine and borane derivatives can attach to the NSO chain to yield a R'3P=N+(R)SOB−R"3 compound. This can happen with tris(tert-butyl) phosphine and tris(pentafluorophenyl)borane. [4]
| Formula | Name | CAS No | PubChem CID | Chemspider ID | MW (g/mol) | Reference |
|---|---|---|---|---|---|---|
| HNSO |
Thionylimide Sulfinylamine Sulfoximine |
13817-04-4 | 139610 | 123125 | 63.074 | [5] |
| C6H5NSO |
N-Sulfinylaniline N-Thionylaniline |
1122-83-4 | 70739 | 63904 | 139.172 | [6] |
| N-Sulfinyl-2,6-diethyl benzenamine | [6] | |||||
| N-Sulfinyl-2-aminopyrimidine | 110526-12-0 | 14790782 | 141.148 | |||
| N-Sulfinyl-n-butylamine | [7] | |||||
| N-Sulfinyl-n-pentylamine | [7] |
- ^ Kresze, G.; Wucherpfennig, W. (1967). "New Methods of Preparative Organic Chemistry V: Organic Syntheses with Imides of Sulfur Dioxide". Angewandte Chemie International Edition in English. 6 (2): 149–167. doi: 10.1002/anie.196701491. PMID 4962859.
- ^ Heravi, Majid M.; Talaei, Bahareh (2014). Ketenes as Privileged Synthons in the Syntheses of Heterocyclic Compounds. Part 1. Advances in Heterocyclic Chemistry. Vol. 113. pp. 143–244. doi: 10.1016/B978-0-12-800170-7.00004-3. ISBN 9780128001707.
- ^ Romano, R.M.; Della Védova, C.O. (2000). "N-Sulfinylimine compounds, R–N=S=O: A chemistry family with strong temperament". Journal of Molecular Structure. 522 (1–3): 1–26. Bibcode: 2000JMoSt.522....1R. doi: 10.1016/S0022-2860(99)00453-6..
- ^ a b Longobardi, Lauren E.; Wolter, Vanessa; Stephan, Douglas W. (12 January 2015). "Frustrated Lewis Pair Activation of an N-Sulfinylamine: A Source of Sulfur Monoxide". Angewandte Chemie International Edition. 54 (3): 809–812. doi: 10.1002/anie.201409969. PMID 25376102.
- ^ Kresze, G.; Maschke, A.; Albrecht, R.; Bederke, K.; Patzschke, H. P.; Smalla, H.; Trede, A. (February 1962). "Organic N-Sulfinyl Compounds". Angewandte Chemie International Edition in English. 1 (2): 89–98. doi: 10.1002/anie.196200891.
- ^ a b Romano, R.M; Della Védova, C.O; Boese, R (January 1999). "A solid state study of the configuration and conformation of OSN–R (R=C6H5 and C6H3(CH3–CH2)2-2,6)". Journal of Molecular Structure. 475 (1): 1–4. Bibcode: 1999JMoSt.475....1R. doi: 10.1016/S0022-2860(98)00439-6.
- ^ a b Ammoscato, Vince (1990). "Part I. A study of the alkylation chemistry of N-sulfinyl amines. Part II. Attempted preparation of the camphor imine of stereospecifically deuterated glycine". Electronic Theses and Dissertations. University of Windsor. Retrieved 28 January 2018.


Sulfinylamines (formerly N-sulfinyl amines) are organosulfur compounds with the formula RNSO where R = an organic substituent. These compounds are, formally speaking, derivatives of HN=S=O, i.e. analogues of sulfur dioxide and of sulfur diimide. A common example is N-sulfinylaniline. Sulfinyl amines are dienophile. [1] They undergo [2+2] cycloaddition to ketenes. [2]
According to X-ray crystallography, sulfinylamines have planar C-N=S=O cores with syn geometry. [3]
Sulfinylamines can be made when thionyl chloride SOCl2 reacts with a primary amine. [4]
Mixtures of phosphine and borane derivatives can attach to the NSO chain to yield a R'3P=N+(R)SOB−R"3 compound. This can happen with tris(tert-butyl) phosphine and tris(pentafluorophenyl)borane. [4]
| Formula | Name | CAS No | PubChem CID | Chemspider ID | MW (g/mol) | Reference |
|---|---|---|---|---|---|---|
| HNSO |
Thionylimide Sulfinylamine Sulfoximine |
13817-04-4 | 139610 | 123125 | 63.074 | [5] |
| C6H5NSO |
N-Sulfinylaniline N-Thionylaniline |
1122-83-4 | 70739 | 63904 | 139.172 | [6] |
| N-Sulfinyl-2,6-diethyl benzenamine | [6] | |||||
| N-Sulfinyl-2-aminopyrimidine | 110526-12-0 | 14790782 | 141.148 | |||
| N-Sulfinyl-n-butylamine | [7] | |||||
| N-Sulfinyl-n-pentylamine | [7] |
- ^ Kresze, G.; Wucherpfennig, W. (1967). "New Methods of Preparative Organic Chemistry V: Organic Syntheses with Imides of Sulfur Dioxide". Angewandte Chemie International Edition in English. 6 (2): 149–167. doi: 10.1002/anie.196701491. PMID 4962859.
- ^ Heravi, Majid M.; Talaei, Bahareh (2014). Ketenes as Privileged Synthons in the Syntheses of Heterocyclic Compounds. Part 1. Advances in Heterocyclic Chemistry. Vol. 113. pp. 143–244. doi: 10.1016/B978-0-12-800170-7.00004-3. ISBN 9780128001707.
- ^ Romano, R.M.; Della Védova, C.O. (2000). "N-Sulfinylimine compounds, R–N=S=O: A chemistry family with strong temperament". Journal of Molecular Structure. 522 (1–3): 1–26. Bibcode: 2000JMoSt.522....1R. doi: 10.1016/S0022-2860(99)00453-6..
- ^ a b Longobardi, Lauren E.; Wolter, Vanessa; Stephan, Douglas W. (12 January 2015). "Frustrated Lewis Pair Activation of an N-Sulfinylamine: A Source of Sulfur Monoxide". Angewandte Chemie International Edition. 54 (3): 809–812. doi: 10.1002/anie.201409969. PMID 25376102.
- ^ Kresze, G.; Maschke, A.; Albrecht, R.; Bederke, K.; Patzschke, H. P.; Smalla, H.; Trede, A. (February 1962). "Organic N-Sulfinyl Compounds". Angewandte Chemie International Edition in English. 1 (2): 89–98. doi: 10.1002/anie.196200891.
- ^ a b Romano, R.M; Della Védova, C.O; Boese, R (January 1999). "A solid state study of the configuration and conformation of OSN–R (R=C6H5 and C6H3(CH3–CH2)2-2,6)". Journal of Molecular Structure. 475 (1): 1–4. Bibcode: 1999JMoSt.475....1R. doi: 10.1016/S0022-2860(98)00439-6.
- ^ a b Ammoscato, Vince (1990). "Part I. A study of the alkylation chemistry of N-sulfinyl amines. Part II. Attempted preparation of the camphor imine of stereospecifically deuterated glycine". Electronic Theses and Dissertations. University of Windsor. Retrieved 28 January 2018.