| |
| Names | |
|---|---|
|
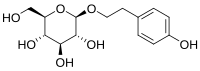
IUPAC name
2-(4-Hydroxyphenyl)ethyl β-D-glucopyranoside
| |
|
Systematic IUPAC name
(2R,3S,4S,5R,6R)-2-(Hydroxymethyl)-6-[2-(4-hydroxyphenyl)ethoxy]oxane-3,4,5-triol | |
| Other names
Salidroside
Rhodioloside Tyrosol glucoside | |
| Identifiers | |
3D model (
JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.224.258 |
PubChem
CID
|
|
| UNII | |
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C14H20O7 | |
| Molar mass | 300.307 g·mol−1 |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Salidroside (rhodioloside) is a glucoside of tyrosol found in the plant Rhodiola rosea. [1] It has been studied, along with rosavin, as one of the potential compounds responsible for the putative antidepressant and anxiolytic actions of this plant. [2] [3] Salidroside may be more active than rosavin, [4] even though many commercially marketed Rhodiola rosea extracts are standardized for rosavin content rather than salidroside.
Bioactivities
Salidroside was shown to improve glucose homeostasis and alleviate diabetic retinopathy in obese mice. [5] [6] The antioxidant, anti-inflammatory and neuroprotective effects of salidroside have also been reported. [7] [8] [9]
Biosynthesis
The salidroside biosynthetic pathway in Rhodiola rosea was described in 2018. [10] Rhodiola contains a pyridoxal phosphate-dependent 4-hydroxyphenylacetaldehyde (4-HPAA) synthase that converts tyrosine to 4-HPAA, which is further reduced to tyrosol by 4-HPAA reductase. Rhodiola contains a regio-selective tyrosol:UDP-glucose 8-O-glucosyltransferase that glycosylates tyrosol to produce salidroside.
References
- ^ Mao Y, Li Y, Yao N (November 2007). "Simultaneous determination of salidroside and tyrosol in extracts of Rhodiola L. by microwave assisted extraction and high-performance liquid chromatography". Journal of Pharmaceutical and Biomedical Analysis. 45 (3): 510–5. doi: 10.1016/j.jpba.2007.05.031. PMID 17628386.
- ^ Perfumi M, Mattioli L (January 2007). "Adaptogenic and central nervous system effects of single doses of 3% rosavin and 1% salidroside Rhodiola rosea L. extract in mice". Phytotherapy Research. 21 (1): 37–43. doi: 10.1002/ptr.2013. PMID 17072830. S2CID 46232827.
- ^ Mattioli L, Funari C, Perfumi M (March 2009). "Effects of Rhodiola rosea L. extract on behavioural and physiological alterations induced by chronic mild stress in female rats". Journal of Psychopharmacology. 23 (2): 130–42. doi: 10.1177/0269881108089872. PMID 18515456. S2CID 206489765.
- ^ Panossian A, Nikoyan N, Ohanyan N, Hovhannisyan A, Abrahamyan H, Gabrielyan E, Wikman G (January 2008). "Comparative study of Rhodiola preparations on behavioral despair of rats". Phytomedicine. 15 (1–2): 84–91. doi: 10.1016/j.phymed.2007.10.003. PMID 18054474.
- ^ Wang, Meihong; Luo, Lan; Yao, Lili; Wang, Caiping; Jiang, Ketao; Liu, Xiaoyu; Xu, Muchen; Shen, Ningmei; Guo, Shaodong; Sun, Cheng; Yang, Yumin (May 2016). "Salidroside improves glucose homeostasis in obese mice by repressing inflammation in white adipose tissues and improving leptin sensitivity in hypothalamus". Scientific Reports. 6 (1): 25399. Bibcode: 2016NatSR...625399W. doi: 10.1038/srep25399. ISSN 2045-2322. PMC 4857131. PMID 27145908.
- ^ Yao, Fei; Jiang, Xinyi; Qiu, Ling; Peng, Zixuan; Zheng, Wei; Ding, Lexi; Xia, Xiaobo (2022). "Long-Term Oral Administration of Salidroside Alleviates Diabetic Retinopathy in db/db Mice". Frontiers in Endocrinology. 13: 861452. doi: 10.3389/fendo.2022.861452. ISSN 1664-2392. PMC 8966089. PMID 35370972.
- ^ Zhong, Zhi-feng; Han, Jing; Zhang, Ji-Zhou; Xiao, Qing; Chen, Jing-yan; Zhang, Kai; Hu, Juan; Chen, Li-dian (2019-12-13). "Neuroprotective Effects of Salidroside on Cerebral Ischemia/Reperfusion-Induced Behavioral Impairment Involves the Dopaminergic System". Frontiers in Pharmacology. 10: 1433. doi: 10.3389/fphar.2019.01433. ISSN 1663-9812. PMC 6923222. PMID 31920641.
- ^ Song, Dan; Zhao, Min; Feng, Liuxiang; Wang, Pingyi; Li, Yimei; Li, Wenhua (October 2021). "Salidroside attenuates acute lung injury via inhibition of inflammatory cytokine production". Biomedicine & Pharmacotherapy. 142: 111949. doi: 10.1016/j.biopha.2021.111949. ISSN 1950-6007. PMID 34325302.
- ^ Ju, Linjie; Wen, Xiaohua; Wang, Chunjun; Wei, Yingjie; Peng, Yunru; Ding, Yongfang; Feng, Liang; Shu, Luan (2017-10-18). "Salidroside, A Natural Antioxidant, Improves β-Cell Survival and Function via Activating AMPK Pathway". Frontiers in Pharmacology. 8. doi: 10.3389/fphar.2017.00749. ISSN 1663-9812. PMC 5651268. PMID 29093682.
- ^ Torrens-Spence, Michael P.; Pluskal, Tomáš; Li, Fu-Shuang; Carballo, Valentina; Weng, Jing-Ke (2018-01-08). "Complete Pathway Elucidation and Heterologous Reconstitution of Rhodiola Salidroside Biosynthesis". Molecular Plant. 11 (1): 205–217. doi: 10.1016/j.molp.2017.12.007. ISSN 1752-9867. PMID 29277428.

| |
| Names | |
|---|---|
|
IUPAC name
2-(4-Hydroxyphenyl)ethyl β-D-glucopyranoside
| |
|
Systematic IUPAC name
(2R,3S,4S,5R,6R)-2-(Hydroxymethyl)-6-[2-(4-hydroxyphenyl)ethoxy]oxane-3,4,5-triol | |
| Other names
Salidroside
Rhodioloside Tyrosol glucoside | |
| Identifiers | |
3D model (
JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.224.258 |
PubChem
CID
|
|
| UNII | |
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C14H20O7 | |
| Molar mass | 300.307 g·mol−1 |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Salidroside (rhodioloside) is a glucoside of tyrosol found in the plant Rhodiola rosea. [1] It has been studied, along with rosavin, as one of the potential compounds responsible for the putative antidepressant and anxiolytic actions of this plant. [2] [3] Salidroside may be more active than rosavin, [4] even though many commercially marketed Rhodiola rosea extracts are standardized for rosavin content rather than salidroside.
Bioactivities
Salidroside was shown to improve glucose homeostasis and alleviate diabetic retinopathy in obese mice. [5] [6] The antioxidant, anti-inflammatory and neuroprotective effects of salidroside have also been reported. [7] [8] [9]
Biosynthesis
The salidroside biosynthetic pathway in Rhodiola rosea was described in 2018. [10] Rhodiola contains a pyridoxal phosphate-dependent 4-hydroxyphenylacetaldehyde (4-HPAA) synthase that converts tyrosine to 4-HPAA, which is further reduced to tyrosol by 4-HPAA reductase. Rhodiola contains a regio-selective tyrosol:UDP-glucose 8-O-glucosyltransferase that glycosylates tyrosol to produce salidroside.
References
- ^ Mao Y, Li Y, Yao N (November 2007). "Simultaneous determination of salidroside and tyrosol in extracts of Rhodiola L. by microwave assisted extraction and high-performance liquid chromatography". Journal of Pharmaceutical and Biomedical Analysis. 45 (3): 510–5. doi: 10.1016/j.jpba.2007.05.031. PMID 17628386.
- ^ Perfumi M, Mattioli L (January 2007). "Adaptogenic and central nervous system effects of single doses of 3% rosavin and 1% salidroside Rhodiola rosea L. extract in mice". Phytotherapy Research. 21 (1): 37–43. doi: 10.1002/ptr.2013. PMID 17072830. S2CID 46232827.
- ^ Mattioli L, Funari C, Perfumi M (March 2009). "Effects of Rhodiola rosea L. extract on behavioural and physiological alterations induced by chronic mild stress in female rats". Journal of Psychopharmacology. 23 (2): 130–42. doi: 10.1177/0269881108089872. PMID 18515456. S2CID 206489765.
- ^ Panossian A, Nikoyan N, Ohanyan N, Hovhannisyan A, Abrahamyan H, Gabrielyan E, Wikman G (January 2008). "Comparative study of Rhodiola preparations on behavioral despair of rats". Phytomedicine. 15 (1–2): 84–91. doi: 10.1016/j.phymed.2007.10.003. PMID 18054474.
- ^ Wang, Meihong; Luo, Lan; Yao, Lili; Wang, Caiping; Jiang, Ketao; Liu, Xiaoyu; Xu, Muchen; Shen, Ningmei; Guo, Shaodong; Sun, Cheng; Yang, Yumin (May 2016). "Salidroside improves glucose homeostasis in obese mice by repressing inflammation in white adipose tissues and improving leptin sensitivity in hypothalamus". Scientific Reports. 6 (1): 25399. Bibcode: 2016NatSR...625399W. doi: 10.1038/srep25399. ISSN 2045-2322. PMC 4857131. PMID 27145908.
- ^ Yao, Fei; Jiang, Xinyi; Qiu, Ling; Peng, Zixuan; Zheng, Wei; Ding, Lexi; Xia, Xiaobo (2022). "Long-Term Oral Administration of Salidroside Alleviates Diabetic Retinopathy in db/db Mice". Frontiers in Endocrinology. 13: 861452. doi: 10.3389/fendo.2022.861452. ISSN 1664-2392. PMC 8966089. PMID 35370972.
- ^ Zhong, Zhi-feng; Han, Jing; Zhang, Ji-Zhou; Xiao, Qing; Chen, Jing-yan; Zhang, Kai; Hu, Juan; Chen, Li-dian (2019-12-13). "Neuroprotective Effects of Salidroside on Cerebral Ischemia/Reperfusion-Induced Behavioral Impairment Involves the Dopaminergic System". Frontiers in Pharmacology. 10: 1433. doi: 10.3389/fphar.2019.01433. ISSN 1663-9812. PMC 6923222. PMID 31920641.
- ^ Song, Dan; Zhao, Min; Feng, Liuxiang; Wang, Pingyi; Li, Yimei; Li, Wenhua (October 2021). "Salidroside attenuates acute lung injury via inhibition of inflammatory cytokine production". Biomedicine & Pharmacotherapy. 142: 111949. doi: 10.1016/j.biopha.2021.111949. ISSN 1950-6007. PMID 34325302.
- ^ Ju, Linjie; Wen, Xiaohua; Wang, Chunjun; Wei, Yingjie; Peng, Yunru; Ding, Yongfang; Feng, Liang; Shu, Luan (2017-10-18). "Salidroside, A Natural Antioxidant, Improves β-Cell Survival and Function via Activating AMPK Pathway". Frontiers in Pharmacology. 8. doi: 10.3389/fphar.2017.00749. ISSN 1663-9812. PMC 5651268. PMID 29093682.
- ^ Torrens-Spence, Michael P.; Pluskal, Tomáš; Li, Fu-Shuang; Carballo, Valentina; Weng, Jing-Ke (2018-01-08). "Complete Pathway Elucidation and Heterologous Reconstitution of Rhodiola Salidroside Biosynthesis". Molecular Plant. 11 (1): 205–217. doi: 10.1016/j.molp.2017.12.007. ISSN 1752-9867. PMID 29277428.