The prodiginines are a family of red tri pyrrole dyestuffs produced by Gammaproteobacteria (e.g. Serratia marcescens) as well as some Actinomycetota (e.g. Streptomyces coelicolor). The group is named after prodigiosin (prodiginine) and is biosynthesized through a common set of enzymes. [1] They are interesting due to their history and their varied biological activity. [2]
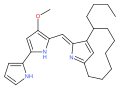
- Prodiginine structures
-
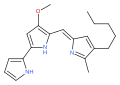
Cycloprodigiosin
-
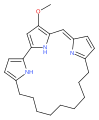
Cyclononylprodigiosin
-
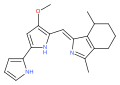
Butyl-meta-cycloheptylprodiginine
The prodiginines are secondary metabolites originally noted in Serratia species, especially Serratia marcescens. They are also found in Actinomycetes, for example Streptomyces coelicolor and some marine bacteria, including Hahella chejuensis and Pseudoalteromonas denitrificans. Cyclononylprodigiosin was isolated from Actinomadura species. [2] [3]
The Prodiginine family consists of primarily red-pigmented tripyrrole secondary metabolites. [4]
 |
 |
Prodiginine, first extracted from terrestrial Serratia marcescens, consisted of a straight alkyl chain substituent and was named prodigiosin.
[5]
The prodiginines are produced from a common intermediate, tambjamine aldehyde (also known as MBC, from its systematic name 4-methoxy-2,2'-bipyrrole-5-carboxaldehyde). This contains two pyrrole rings built from proline and serine as shown in the blue-shaded pathway in Figure 1. [1] The aldehyde is subsequently condensed with a third pyrrole to form prodigiosin [6] (compound 16 in Figure 1), which is then further elaborated to cycloprodigiosin (compound 22 in Figure 1) and the other members of the chemical family. [2] [7] [8]
Details of the first total synthesis of the parent prodigiosin were published in 1962, confirming the chemical structure. As with the biosynthesis, the key intermediate was MBC. [9] This aldehyde has subsequently been prepared by other methods and used to make many prodiginines. [8]
Prodigiosin was considered for commercial production in 1823 to dye silk and wool but it has poor stability to light and the advent of synthetic alternatives cut short this application. [8] The group has also been investigated for its pharmaceutical potential as anticancer, immunosuppressant, and antimalarial agents. [2] [3] [10]
- Serratia marcescens, especially for the history of prodigiosin's discovery
- ^ a b c Sakai-Kawada, Francis E.; Ip, Courtney G.; Hagiwara, Kehau A.; Awaya, Jonathan D. (2019). "Biosynthesis and Bioactivity of Prodiginine Analogs in Marine Bacteria, Pseudoalteromonas: A Mini Review". Frontiers in Microbiology. 10: 1715. doi: 10.3389/fmicb.2019.01715. PMC 6667630. PMID 31396200.
- ^ a b c d Williamson NR, Fineran PC, Gristwood T, Leeper FJ, Salmond GP (2006). "The biosynthesis and regulation of bacterial prodiginines". Nature Reviews Microbiology. 4 (12): 887–899. doi: 10.1038/nrmicro1531. PMID 17109029. S2CID 11649828.
- ^ a b Bennett, J.W.; Bentley, Ronald (2000). Seeing red: The story of prodigiosin. Advances in Applied Microbiology. Vol. 47. pp. 1–32. doi: 10.1016/S0065-2164(00)47000-0. ISBN 9780120026470. PMID 12876793.
- ^ Sakai-Kawada, Francis E.; Ip, Courtney G.; Hagiwara, Kehau A.; Awaya, Jonathan D. (2019). "Biosynthesis and Bioactivity of Prodiginine Analogs in Marine Bacteria, Pseudoalteromonas: A Mini Review". Frontiers in Microbiology. 10. doi: 10.3389/fmicb.2019.01715. ISSN 1664-302X. PMC 6667630. PMID 31396200.
- ^ Rapoport, Henry.; Holden, Kenneth G. (1962-02-01). "The Synthesis of Prodigiosin". Journal of the American Chemical Society. 84 (4): 635–642. doi: 10.1021/ja00863a026. ISSN 0002-7863.
- ^ R. Caspi (2014-08-14). "Pathway: prodigiosin biosynthesis". MetaCyc Metabolic Pathway Database. Retrieved 2021-04-01.
- ^ Walsh, Christopher T.; Garneau-Tsodikova, Sylvie; Howard-Jones, Annaleise R. (2006). "Biological formation of pyrroles: Nature's logic and enzymatic machinery". Natural Product Reports. 23 (4): 517–31. doi: 10.1039/B605245M. PMID 16874387.
- ^ a b c Hu, Dennis X.; Withall, David M.; Challis, Gregory L.; Thomson, Regan J. (2016). "Structure, Chemical Synthesis, and Biosynthesis of Prodiginine Natural Products". Chemical Reviews. 116 (14): 7818–7853. doi: 10.1021/acs.chemrev.6b00024. PMC 5555159. PMID 27314508.
- ^ Rapoport, Henry.; Willson, Clyde D. (1962). "The Preparation and Properties of Some Methoxypyrroles". Journal of the American Chemical Society. 84 (4): 630–635. doi: 10.1021/ja00863a025.
- ^ Stankovic, Nada; Senerovic, Lidija; Ilic-Tomic, Tatjana; Vasiljevic, Branka; Nikodinovic-Runic, Jasmina (2014). "Properties and applications of undecylprodigiosin and other bacterial prodigiosins". Applied Microbiology and Biotechnology. 98 (9): 3841–3858. doi: 10.1007/s00253-014-5590-1. PMID 24562326. S2CID 16834175.

The prodiginines are a family of red tri pyrrole dyestuffs produced by Gammaproteobacteria (e.g. Serratia marcescens) as well as some Actinomycetota (e.g. Streptomyces coelicolor). The group is named after prodigiosin (prodiginine) and is biosynthesized through a common set of enzymes. [1] They are interesting due to their history and their varied biological activity. [2]
- Prodiginine structures
-
Cycloprodigiosin
-
Cyclononylprodigiosin
-
Butyl-meta-cycloheptylprodiginine
The prodiginines are secondary metabolites originally noted in Serratia species, especially Serratia marcescens. They are also found in Actinomycetes, for example Streptomyces coelicolor and some marine bacteria, including Hahella chejuensis and Pseudoalteromonas denitrificans. Cyclononylprodigiosin was isolated from Actinomadura species. [2] [3]
The Prodiginine family consists of primarily red-pigmented tripyrrole secondary metabolites. [4]
 |
 |
Prodiginine, first extracted from terrestrial Serratia marcescens, consisted of a straight alkyl chain substituent and was named prodigiosin.
[5]
The prodiginines are produced from a common intermediate, tambjamine aldehyde (also known as MBC, from its systematic name 4-methoxy-2,2'-bipyrrole-5-carboxaldehyde). This contains two pyrrole rings built from proline and serine as shown in the blue-shaded pathway in Figure 1. [1] The aldehyde is subsequently condensed with a third pyrrole to form prodigiosin [6] (compound 16 in Figure 1), which is then further elaborated to cycloprodigiosin (compound 22 in Figure 1) and the other members of the chemical family. [2] [7] [8]
Details of the first total synthesis of the parent prodigiosin were published in 1962, confirming the chemical structure. As with the biosynthesis, the key intermediate was MBC. [9] This aldehyde has subsequently been prepared by other methods and used to make many prodiginines. [8]
Prodigiosin was considered for commercial production in 1823 to dye silk and wool but it has poor stability to light and the advent of synthetic alternatives cut short this application. [8] The group has also been investigated for its pharmaceutical potential as anticancer, immunosuppressant, and antimalarial agents. [2] [3] [10]
- Serratia marcescens, especially for the history of prodigiosin's discovery
- ^ a b c Sakai-Kawada, Francis E.; Ip, Courtney G.; Hagiwara, Kehau A.; Awaya, Jonathan D. (2019). "Biosynthesis and Bioactivity of Prodiginine Analogs in Marine Bacteria, Pseudoalteromonas: A Mini Review". Frontiers in Microbiology. 10: 1715. doi: 10.3389/fmicb.2019.01715. PMC 6667630. PMID 31396200.
- ^ a b c d Williamson NR, Fineran PC, Gristwood T, Leeper FJ, Salmond GP (2006). "The biosynthesis and regulation of bacterial prodiginines". Nature Reviews Microbiology. 4 (12): 887–899. doi: 10.1038/nrmicro1531. PMID 17109029. S2CID 11649828.
- ^ a b Bennett, J.W.; Bentley, Ronald (2000). Seeing red: The story of prodigiosin. Advances in Applied Microbiology. Vol. 47. pp. 1–32. doi: 10.1016/S0065-2164(00)47000-0. ISBN 9780120026470. PMID 12876793.
- ^ Sakai-Kawada, Francis E.; Ip, Courtney G.; Hagiwara, Kehau A.; Awaya, Jonathan D. (2019). "Biosynthesis and Bioactivity of Prodiginine Analogs in Marine Bacteria, Pseudoalteromonas: A Mini Review". Frontiers in Microbiology. 10. doi: 10.3389/fmicb.2019.01715. ISSN 1664-302X. PMC 6667630. PMID 31396200.
- ^ Rapoport, Henry.; Holden, Kenneth G. (1962-02-01). "The Synthesis of Prodigiosin". Journal of the American Chemical Society. 84 (4): 635–642. doi: 10.1021/ja00863a026. ISSN 0002-7863.
- ^ R. Caspi (2014-08-14). "Pathway: prodigiosin biosynthesis". MetaCyc Metabolic Pathway Database. Retrieved 2021-04-01.
- ^ Walsh, Christopher T.; Garneau-Tsodikova, Sylvie; Howard-Jones, Annaleise R. (2006). "Biological formation of pyrroles: Nature's logic and enzymatic machinery". Natural Product Reports. 23 (4): 517–31. doi: 10.1039/B605245M. PMID 16874387.
- ^ a b c Hu, Dennis X.; Withall, David M.; Challis, Gregory L.; Thomson, Regan J. (2016). "Structure, Chemical Synthesis, and Biosynthesis of Prodiginine Natural Products". Chemical Reviews. 116 (14): 7818–7853. doi: 10.1021/acs.chemrev.6b00024. PMC 5555159. PMID 27314508.
- ^ Rapoport, Henry.; Willson, Clyde D. (1962). "The Preparation and Properties of Some Methoxypyrroles". Journal of the American Chemical Society. 84 (4): 630–635. doi: 10.1021/ja00863a025.
- ^ Stankovic, Nada; Senerovic, Lidija; Ilic-Tomic, Tatjana; Vasiljevic, Branka; Nikodinovic-Runic, Jasmina (2014). "Properties and applications of undecylprodigiosin and other bacterial prodigiosins". Applied Microbiology and Biotechnology. 98 (9): 3841–3858. doi: 10.1007/s00253-014-5590-1. PMID 24562326. S2CID 16834175.