The polyiodides are a class of
polyhalogen anions composed entirely of
iodine atoms.
[1]
[2] The most common member is the
triiodide ion, I−
3. Other known larger polyiodides include [I42−, [I5−, [I62−, [I7−, [I82−, [I9−, [I102−, [I104−, [I113−, [I122−, [I133−, [I144-, [I162−, [I224−, [I263−, [I264−, [I284− and [I293−. All these can be considered as formed from the interaction of the I–, I2, and I−
3 building blocks.
The polyiodides can be made by addition of stoichiometric amounts of I2 to solutions containing I− and I−
3, with the presence of large
countercations to stabilize them. For example, KI3·H2O can be crystallized from a saturated solution of
KI when a stoichiometric amount of I2 is added and cooled.
[3]
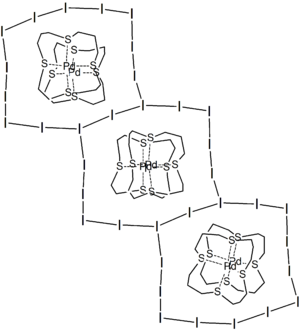

Polyiodides adopt diverse structures. Most can be considered as associations of I2, I−, and I−
3 units. Discrete polyiodides are usually
linear. The more complex two- or three-dimensional network structures of chains and cages are formed as the ions interact with each other, with their shapes depending on their associated
cations quite strongly, a phenomenon named dimensional caging.
[4]
[5] The table below lists the polyiodide salts which have been structurally characterized, along with their counter-cation.
[6]
| Anion | Counter-cation | Structural description |
|---|---|---|
| [I2− | Na(C3H6O)+ 3 |
linear [7] [8] |
| [I3− | Cs+, (C4H9)4N+ | linear |
| [I42− | [Cu(NH3)42+ | symmetric linear array of iodine atoms [9] |
| [I5− | [EtMe3N]+ | V-shaped with polymeric layers |
| [EtMePh2N]+ | V-shaped with isolated [I5− ions | |
| [I62− | [NH3(CH2)8NH32+ | almost linear [ [10]] |
| [I7− | [Ag( 18aneS6)]+ | an anionic network derived from a primitive rhombohedral lattice of iodide ions bridged by I2 molecules |
| [I82− | [Ni( phen)32+ | regular anionic shapes, can be described as [I− 3·I2·I− 8] or [I− 3·I− 5] |
| [I9− | [Me2 iPr PhN]+ | 14-membered ring tied by two I2 bridges to give 10-membered rings |
| [Me4N]+ | non-octahedral, but a twisted "h"-like arrangement of I− 3 and I2 units | |
| [I102− | [Cd( 12-crown-4)22+; Theophyllinium | twisted ring configuration with two I− 3 units linked by two I2 molecules [11] |
| [I113− | [( 16aneS4) PdIPd(16aneS4)]3+ | 14-membered ring (9.66 × 12.64 Å) around the complex cation, with the rings interlink further to give an infinite 2D sheet |
| [I122− | [Ag2( 15aneS5)22+ | extended 3D spiral superstructure supported by Ag–I bonds and weak I···S interactions |
| [Cu( Dafone)32+ | planar configuration | |
| [I133− | [Me2Ph2N]+ | consists of zigzag chains of I− and I2 |
| [I144− | 4,4′-bipyridinium | double hook (I− 3·I2·I−·I2·I−·I2·I− 3) [12] |
| [I162− | [Me2Ph2N]+ | centrosymmetric arrangement of [I− 7·I2·I− 7] |
| iPrMe2PhN]+ | the anion forms 14-membered rings catenated by I2 molecules, which further link into layers with 10- and 14-membered rings | |
| [I224− | [MePh3P]+ | two L-shaped [I5− units linked by an I2 molecule and completed by two end-on [I5− groups |
| [I263− | [Me3S]+ | consists of [I5− and [I7− ions with intercalated I2 molecules |
| [I264− | Cp*2Fe+ | an anionic network derived from a primitive cubic lattice built from I− ions, with I2 bridges on all edges and systematically removing 1⁄12 of the I2 molecules |
| [I293− | Cp2Fe+ | an anionic 3D network with a cage-like structure of [{(I− 5)1⁄2·I2}·{(I2− 12)1⁄2·I2}·I2], with [Cp2Fe]+ ions interacting with the anion in the cavities [13] |
| [I∞δ− | Pyrroloperylene+• | Infinite polyiodide homopolymer. [14] |

Polyiodide compounds are generally sensitive to light.
Triiodide, I−
3, undergoes unimolecular
photodissociation.
[15]
[16] Polyiodide has been used to improve the scalability in the synthesis of halide
perovskite
photovoltaic materials.
[17]
Solid state compounds containing linear-chain polyiodide ions exhibit enhanced conductivity [18] [19] than their simple iodide counterparts. The conductivity can be drastically modified by external pressure, which changes the interatomic distances between iodine moieties and the charge distribution. [20]
- Triiodide
- Polyhalogen ions
- Iodine–starch test
- Dye-sensitized solar cell
- Halogen bond
- Catenation
- Inorganic polymer
- ^ Housecroft, Catherine E.; Sharpe, Alan G. (2008). "Chapter 17: The group 17 elements". Inorganic Chemistry (3rd ed.). Pearson. p. 547. ISBN 978-0-13-175553-6.
- ^ Kloo, Lars (2021), "Catenated compounds in Group 17—Polyhalides", Reference Module in Chemistry, Molecular Sciences and Chemical Engineering, Elsevier, pp. 1021–1049, doi: 10.1016/b978-0-12-823144-9.00013-3, ISBN 978-0-12-409547-2, S2CID 242567501, retrieved 2022-03-28
- ^ Brauer, G., ed. (1963). "Potassium triiodide". Handbook of Preparative Inorganic Chemistry. Vol. 1 (2nd ed.). New York: Academic Press. p. 294.
- ^ Svensson, Per H.; Gorlov, Mikhail; Kloo, Lars (2008-12-15). "Dimensional Caging of Polyiodides". Inorganic Chemistry. 47 (24): 11464–11466. doi: 10.1021/ic801820s. ISSN 0020-1669. PMID 19053351.
- ^ García, Marcos D.; Martí-Rujas, Javier; Metrangolo, Pierangelo; Peinador, Carlos; Pilati, Tullio; Resnati, Giuseppe; Terraneo, Giancarlo; Ursini, Maurizio (2011). "Dimensional caging of polyiodides: cation-templated synthesis using bipyridinium salts". CrystEngComm. 13 (13): 4411. doi: 10.1039/c0ce00860e. ISSN 1466-8033.
- ^ King, R. Bruce (2005). "Chlorine, Bromine, Iodine, & Astatine: Inorganic Chemistry". Encyclopedia of Inorganic Chemistry (2nd ed.). Wiley. p. 747. ISBN 9780470862100.
- ^ Rzepa, Henry (May 16, 2009). "The mystery of the Finkelstein reaction". Chemistry with a twist.
- ^ Howie, R. Alan; Wardell, James L. (2003-05-15). "Polymeric tris(μ2-acetone-κ2O:O)sodium polyiodide at 120 K". Acta Crystallographica Section C Crystal Structure Communications. 59 (5): m184–m186. doi: 10.1107/S0108270103006395. ISSN 0108-2701. PMID 12743392.
- ^ Svensson, Per H.; Kloo, Lars (2003). "Synthesis, Structure, and Bonding in Polyiodide and Metal Iodide–Iodine Systems". Chem. Rev. 103 (5): 1649–84. doi: 10.1021/cr0204101. PMID 12744691.
- ^ Reiss, Guido J.; Van Megen, Martin (2013). "I62− Anion Composed of Two Asymmetric Triiodide Moieties: A Competition between Halogen and Hydrogen Bond". Inorganics. 1 (1): 3–13. doi: 10.3390/inorganics1010003.
- ^ Reiss, Guido J. (2019-06-26). "A cyclic I102− anion in the layered crystal structure of theophyllinium pentaiodide, C7H9I5N4O2". Zeitschrift für Kristallographie – New Crystal Structures. 234 (4): 737–739. doi: 10.1515/ncrs-2019-0082. ISSN 2197-4578.
- ^ Reiss, Guido J.; Megen, Martin van (2012). "Two New Polyiodides in the 4,4′-Bipyridinium Diiodide/Iodine System". Zeitschrift für Naturforschung B. 67 (1): 5–10. doi: 10.1515/znb-2012-0102. ISSN 1865-7117. S2CID 5857644.
- ^ Tebbe, Karl-Friedrich; Buchem, Rita (1997-06-16). "Das bisher iodreichste Polyiodid: Herstellung und Struktur von Fc3I29". Angewandte Chemie (in German). 109 (12): 1403–1405. Bibcode: 1997AngCh.109.1403T. doi: 10.1002/ange.19971091233.
- ^ Madhu, Sheri; Evans, Hayden A.; Doan-Nguyen, Vicky V. T.; Labram, John G.; Wu, Guang; Chabinyc, Michael L.; Seshadri, Ram; Wudl, Fred (4 July 2016). "Infinite Polyiodide Chains in the Pyrroloperylene–Iodine Complex: Insights into the Starch-Iodine and Perylene-Iodine Complexes". Angewandte Chemie International Edition. 55 (28): 8032–8035. doi: 10.1002/anie.201601585. PMID 27239781. S2CID 30407996.
- ^ Hoops, Alexandra A.; Gascooke, Jason R.; Faulhaber, Ann Elise; Kautzman, Kathryn E.; Neumark, Daniel M. (May 2004). "Two- and three-body photodissociation of gas phase I3−". The Journal of Chemical Physics. 120 (17): 7901–7909. doi: 10.1063/1.1691017. hdl: 2440/34955. ISSN 0021-9606. PMID 15267705.
- ^ Nakanishi, Ryuzo; Saitou, Naoya; Ohno, Tomoyo; Kowashi, Satomi; Yabushita, Satoshi; Nagata, Takashi (2007-05-28). "Photodissociation of gas-phase I3−: Comprehensive understanding of nonadiabatic dissociation dynamics". The Journal of Chemical Physics. 126 (20): 204311. doi: 10.1063/1.2736691. ISSN 0021-9606. PMID 17552766.
- ^ Turkevych, Ivan; Kazaoui, Said; Belich, Nikolai A.; Grishko, Aleksei Y.; Fateev, Sergey A.; Petrov, Andrey A.; Urano, Toshiyuki; Aramaki, Shinji; Kosar, Sonya; Kondo, Michio; Goodilin, Eugene A. (January 2019). "Strategic advantages of reactive polyiodide melts for scalable perovskite photovoltaics". Nature Nanotechnology. 14 (1): 57–63. doi: 10.1038/s41565-018-0304-y. ISSN 1748-3395. PMID 30478274. S2CID 53784226.
- ^ Alvarez, Santiago; Novoa, Juan; Mota, Fernando (1986-12-26). "The mechanism of electrical conductivity along polyhalide chains". Chemical Physics Letters. 132 (6): 531–534. doi: 10.1016/0009-2614(86)87118-4.
- ^ Yu, Hongtao; Yan, Lijia; He, Yaowu; Meng, Hong; Huang, Wei (2017). "An unusual photoconductive property of polyiodide and enhancement by catenating with 3-thiophenemethylamine salt". Chemical Communications. 53 (2): 432–435. doi: 10.1039/C6CC08595D. ISSN 1359-7345. PMID 27965990.
- ^ Poręba, Tomasz; Ernst, Michelle; Zimmer, Dominik; Macchi, Piero; Casati, Nicola (2019-05-13). "Pressure-Induced Polymerization and Electrical Conductivity of a Polyiodide". Angewandte Chemie International Edition. 58 (20): 6625–6629. doi: 10.1002/anie.201901178. ISSN 1433-7851. PMID 30844119. S2CID 73514885.
The polyiodides are a class of
polyhalogen anions composed entirely of
iodine atoms.
[1]
[2] The most common member is the
triiodide ion, I−
3. Other known larger polyiodides include [I42−, [I5−, [I62−, [I7−, [I82−, [I9−, [I102−, [I104−, [I113−, [I122−, [I133−, [I144-, [I162−, [I224−, [I263−, [I264−, [I284− and [I293−. All these can be considered as formed from the interaction of the I–, I2, and I−
3 building blocks.
The polyiodides can be made by addition of stoichiometric amounts of I2 to solutions containing I− and I−
3, with the presence of large
countercations to stabilize them. For example, KI3·H2O can be crystallized from a saturated solution of
KI when a stoichiometric amount of I2 is added and cooled.
[3]
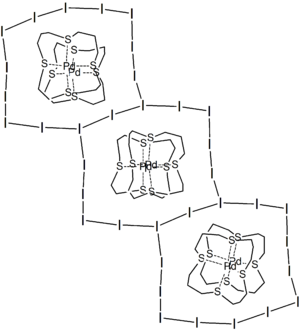

Polyiodides adopt diverse structures. Most can be considered as associations of I2, I−, and I−
3 units. Discrete polyiodides are usually
linear. The more complex two- or three-dimensional network structures of chains and cages are formed as the ions interact with each other, with their shapes depending on their associated
cations quite strongly, a phenomenon named dimensional caging.
[4]
[5] The table below lists the polyiodide salts which have been structurally characterized, along with their counter-cation.
[6]
| Anion | Counter-cation | Structural description |
|---|---|---|
| [I2− | Na(C3H6O)+ 3 |
linear [7] [8] |
| [I3− | Cs+, (C4H9)4N+ | linear |
| [I42− | [Cu(NH3)42+ | symmetric linear array of iodine atoms [9] |
| [I5− | [EtMe3N]+ | V-shaped with polymeric layers |
| [EtMePh2N]+ | V-shaped with isolated [I5− ions | |
| [I62− | [NH3(CH2)8NH32+ | almost linear [ [10]] |
| [I7− | [Ag( 18aneS6)]+ | an anionic network derived from a primitive rhombohedral lattice of iodide ions bridged by I2 molecules |
| [I82− | [Ni( phen)32+ | regular anionic shapes, can be described as [I− 3·I2·I− 8] or [I− 3·I− 5] |
| [I9− | [Me2 iPr PhN]+ | 14-membered ring tied by two I2 bridges to give 10-membered rings |
| [Me4N]+ | non-octahedral, but a twisted "h"-like arrangement of I− 3 and I2 units | |
| [I102− | [Cd( 12-crown-4)22+; Theophyllinium | twisted ring configuration with two I− 3 units linked by two I2 molecules [11] |
| [I113− | [( 16aneS4) PdIPd(16aneS4)]3+ | 14-membered ring (9.66 × 12.64 Å) around the complex cation, with the rings interlink further to give an infinite 2D sheet |
| [I122− | [Ag2( 15aneS5)22+ | extended 3D spiral superstructure supported by Ag–I bonds and weak I···S interactions |
| [Cu( Dafone)32+ | planar configuration | |
| [I133− | [Me2Ph2N]+ | consists of zigzag chains of I− and I2 |
| [I144− | 4,4′-bipyridinium | double hook (I− 3·I2·I−·I2·I−·I2·I− 3) [12] |
| [I162− | [Me2Ph2N]+ | centrosymmetric arrangement of [I− 7·I2·I− 7] |
| iPrMe2PhN]+ | the anion forms 14-membered rings catenated by I2 molecules, which further link into layers with 10- and 14-membered rings | |
| [I224− | [MePh3P]+ | two L-shaped [I5− units linked by an I2 molecule and completed by two end-on [I5− groups |
| [I263− | [Me3S]+ | consists of [I5− and [I7− ions with intercalated I2 molecules |
| [I264− | Cp*2Fe+ | an anionic network derived from a primitive cubic lattice built from I− ions, with I2 bridges on all edges and systematically removing 1⁄12 of the I2 molecules |
| [I293− | Cp2Fe+ | an anionic 3D network with a cage-like structure of [{(I− 5)1⁄2·I2}·{(I2− 12)1⁄2·I2}·I2], with [Cp2Fe]+ ions interacting with the anion in the cavities [13] |
| [I∞δ− | Pyrroloperylene+• | Infinite polyiodide homopolymer. [14] |

Polyiodide compounds are generally sensitive to light.
Triiodide, I−
3, undergoes unimolecular
photodissociation.
[15]
[16] Polyiodide has been used to improve the scalability in the synthesis of halide
perovskite
photovoltaic materials.
[17]
Solid state compounds containing linear-chain polyiodide ions exhibit enhanced conductivity [18] [19] than their simple iodide counterparts. The conductivity can be drastically modified by external pressure, which changes the interatomic distances between iodine moieties and the charge distribution. [20]
- Triiodide
- Polyhalogen ions
- Iodine–starch test
- Dye-sensitized solar cell
- Halogen bond
- Catenation
- Inorganic polymer
- ^ Housecroft, Catherine E.; Sharpe, Alan G. (2008). "Chapter 17: The group 17 elements". Inorganic Chemistry (3rd ed.). Pearson. p. 547. ISBN 978-0-13-175553-6.
- ^ Kloo, Lars (2021), "Catenated compounds in Group 17—Polyhalides", Reference Module in Chemistry, Molecular Sciences and Chemical Engineering, Elsevier, pp. 1021–1049, doi: 10.1016/b978-0-12-823144-9.00013-3, ISBN 978-0-12-409547-2, S2CID 242567501, retrieved 2022-03-28
- ^ Brauer, G., ed. (1963). "Potassium triiodide". Handbook of Preparative Inorganic Chemistry. Vol. 1 (2nd ed.). New York: Academic Press. p. 294.
- ^ Svensson, Per H.; Gorlov, Mikhail; Kloo, Lars (2008-12-15). "Dimensional Caging of Polyiodides". Inorganic Chemistry. 47 (24): 11464–11466. doi: 10.1021/ic801820s. ISSN 0020-1669. PMID 19053351.
- ^ García, Marcos D.; Martí-Rujas, Javier; Metrangolo, Pierangelo; Peinador, Carlos; Pilati, Tullio; Resnati, Giuseppe; Terraneo, Giancarlo; Ursini, Maurizio (2011). "Dimensional caging of polyiodides: cation-templated synthesis using bipyridinium salts". CrystEngComm. 13 (13): 4411. doi: 10.1039/c0ce00860e. ISSN 1466-8033.
- ^ King, R. Bruce (2005). "Chlorine, Bromine, Iodine, & Astatine: Inorganic Chemistry". Encyclopedia of Inorganic Chemistry (2nd ed.). Wiley. p. 747. ISBN 9780470862100.
- ^ Rzepa, Henry (May 16, 2009). "The mystery of the Finkelstein reaction". Chemistry with a twist.
- ^ Howie, R. Alan; Wardell, James L. (2003-05-15). "Polymeric tris(μ2-acetone-κ2O:O)sodium polyiodide at 120 K". Acta Crystallographica Section C Crystal Structure Communications. 59 (5): m184–m186. doi: 10.1107/S0108270103006395. ISSN 0108-2701. PMID 12743392.
- ^ Svensson, Per H.; Kloo, Lars (2003). "Synthesis, Structure, and Bonding in Polyiodide and Metal Iodide–Iodine Systems". Chem. Rev. 103 (5): 1649–84. doi: 10.1021/cr0204101. PMID 12744691.
- ^ Reiss, Guido J.; Van Megen, Martin (2013). "I62− Anion Composed of Two Asymmetric Triiodide Moieties: A Competition between Halogen and Hydrogen Bond". Inorganics. 1 (1): 3–13. doi: 10.3390/inorganics1010003.
- ^ Reiss, Guido J. (2019-06-26). "A cyclic I102− anion in the layered crystal structure of theophyllinium pentaiodide, C7H9I5N4O2". Zeitschrift für Kristallographie – New Crystal Structures. 234 (4): 737–739. doi: 10.1515/ncrs-2019-0082. ISSN 2197-4578.
- ^ Reiss, Guido J.; Megen, Martin van (2012). "Two New Polyiodides in the 4,4′-Bipyridinium Diiodide/Iodine System". Zeitschrift für Naturforschung B. 67 (1): 5–10. doi: 10.1515/znb-2012-0102. ISSN 1865-7117. S2CID 5857644.
- ^ Tebbe, Karl-Friedrich; Buchem, Rita (1997-06-16). "Das bisher iodreichste Polyiodid: Herstellung und Struktur von Fc3I29". Angewandte Chemie (in German). 109 (12): 1403–1405. Bibcode: 1997AngCh.109.1403T. doi: 10.1002/ange.19971091233.
- ^ Madhu, Sheri; Evans, Hayden A.; Doan-Nguyen, Vicky V. T.; Labram, John G.; Wu, Guang; Chabinyc, Michael L.; Seshadri, Ram; Wudl, Fred (4 July 2016). "Infinite Polyiodide Chains in the Pyrroloperylene–Iodine Complex: Insights into the Starch-Iodine and Perylene-Iodine Complexes". Angewandte Chemie International Edition. 55 (28): 8032–8035. doi: 10.1002/anie.201601585. PMID 27239781. S2CID 30407996.
- ^ Hoops, Alexandra A.; Gascooke, Jason R.; Faulhaber, Ann Elise; Kautzman, Kathryn E.; Neumark, Daniel M. (May 2004). "Two- and three-body photodissociation of gas phase I3−". The Journal of Chemical Physics. 120 (17): 7901–7909. doi: 10.1063/1.1691017. hdl: 2440/34955. ISSN 0021-9606. PMID 15267705.
- ^ Nakanishi, Ryuzo; Saitou, Naoya; Ohno, Tomoyo; Kowashi, Satomi; Yabushita, Satoshi; Nagata, Takashi (2007-05-28). "Photodissociation of gas-phase I3−: Comprehensive understanding of nonadiabatic dissociation dynamics". The Journal of Chemical Physics. 126 (20): 204311. doi: 10.1063/1.2736691. ISSN 0021-9606. PMID 17552766.
- ^ Turkevych, Ivan; Kazaoui, Said; Belich, Nikolai A.; Grishko, Aleksei Y.; Fateev, Sergey A.; Petrov, Andrey A.; Urano, Toshiyuki; Aramaki, Shinji; Kosar, Sonya; Kondo, Michio; Goodilin, Eugene A. (January 2019). "Strategic advantages of reactive polyiodide melts for scalable perovskite photovoltaics". Nature Nanotechnology. 14 (1): 57–63. doi: 10.1038/s41565-018-0304-y. ISSN 1748-3395. PMID 30478274. S2CID 53784226.
- ^ Alvarez, Santiago; Novoa, Juan; Mota, Fernando (1986-12-26). "The mechanism of electrical conductivity along polyhalide chains". Chemical Physics Letters. 132 (6): 531–534. doi: 10.1016/0009-2614(86)87118-4.
- ^ Yu, Hongtao; Yan, Lijia; He, Yaowu; Meng, Hong; Huang, Wei (2017). "An unusual photoconductive property of polyiodide and enhancement by catenating with 3-thiophenemethylamine salt". Chemical Communications. 53 (2): 432–435. doi: 10.1039/C6CC08595D. ISSN 1359-7345. PMID 27965990.
- ^ Poręba, Tomasz; Ernst, Michelle; Zimmer, Dominik; Macchi, Piero; Casati, Nicola (2019-05-13). "Pressure-Induced Polymerization and Electrical Conductivity of a Polyiodide". Angewandte Chemie International Edition. 58 (20): 6625–6629. doi: 10.1002/anie.201901178. ISSN 1433-7851. PMID 30844119. S2CID 73514885.