A photoswitch is a type of molecule that can change its structural geometry and chemical properties upon irradiation with electromagnetic radiation. Although often used interchangeably with the term molecular machine, a switch does not perform work upon a change in its shape whereas a machine does. [1] However, photochromic compounds are the necessary building blocks for light driven molecular motors and machines. [2] Upon irradiation with light, photoisomerization about double bonds in the molecule can lead to changes in the cis- or trans- configuration. [3] These photochromic molecules are being considered for a range of applications.
Chemical structures and properties

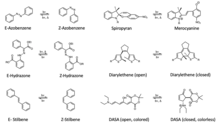
A photochromic compound can change its configuration or structure upon irradiation with light. Several examples of photochromic compounds include: azobenzene, [6] spiropyran, [7] merocyanine, [8] diarylethene, [9] spirooxazine, [10] fulgide, [11] hydrazone, [12] nobormadiene, [13] thioindigo, [14] acrylamide-azobenzene-quaternary ammonia, [15] donor-acceptor Stenhouse adducts, [16] [17] stilbene, [18] etc.
Isomerization
Upon isomerization from the absorption of light, a π-to-π* or n-to-π* electronic transition can occur with the subsequent release of light ( fluorescence or phosphorescence) or heat when electrons transit from an excited state to a ground state. A photostationary state can be achieved when the irradiation of light no longer converts one form of an isomer into another; however, a mixture of cis- and trans- isomers will always exist with a higher percentage of one versus the other depending on the photoconditions. [19]
Mechanism
Although the mechanism for photoisomerization is still debated amongst most scientists, increasing evidence supports cis-/trans- isomerization of polyenes favoring the hula twist rather than the one-bond-flip. [20] The one-bond-flip isomerizes at the reactive double bond while the hula twist undergoes a conformational isomerization at the adjacent single bond. However, the interconversion of stereoisomers of stilbene proceeds via one-bond-flip. [21]
Quantum yield
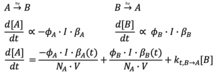
One of the most important properties of a photoswitch is its quantum yield which measures the effectiveness of absorbed light to induce photoisomerization. Quantum yield is modeled and calculated using Arrhenius kinetics. [22] Photoswitches can be in solution or in the solid state; however, switching in the solid state is more difficult to observe due to the lack of molecular freedom of motion, solid packing, and the fast thermal reversion to the ground state. [23] Through chemical modification, red shifting the wavelengths of absorption needed to cause isomerizaiton leads to low light induced switching which has applications in photopharmacology. [24]
Catalysis
When a photochromic compound is incorporated into a suitable catalytic molecule, photoswitchable catalysis can result from the reversible changes in geometric conformation upon irradiation with light. As one of the most widely studied photoswitches, azobenzene has been shown to be an effective switch for regulating catalytic activity due to its isomerization from the E to Z conformation with light, and its ability to thermally relax back to the E isomer in dark conditions. [25]
Biological

Rhodopsins
One of the more prevalent biological examples in the human body that undergoes structural changes upon light irradiation includes the class of membrane-bound photoreceptors, Rhodopsins. [27] These include the regulation of melanocytes, vision, the release of melatonin and the control of the circadian rhythm, etc. [28] Rhodopsins are highly efficient photochromic compounds that can undergo fast photoisomerization and are associated with various retinal proteins [29] along with light-gated channels and pumps in microbes. [30]
Research
Advances in vision restoration with photochromic compounds has been investigated. Fast isomerization allows retinal cells to turn on when activated by light and advances in acrylamide-azobenzene-quaternary ammonia have shown restoration of visual responses in blind mice. [31] Companies involved in this area include Novartis, Vedere, Allergan, and Nanoscope Therapeutics. [32]
Through the incorporation of photoswitches into biological molecules, biological processes can be regulated through controlled irradiation with light. This includes photocontrol of peptide conformation and activity, transcription and translation of DNA and RNA, regulation of enzymatic activity, and photoregulated ion channels. [33] For example, optical control of ligand binding in human serum albumin has been demonstrated to influence its allosteric binding properties. [34] Also, red-shifted azobenzenes have been used to control ionotropic glutamate receptors. [35]
Potential applications
Photoswitches are studied in biology, materials chemistry, and physics and have a wide variety of potential applications, especially in the framework of nanotechnology. [36]
Electronics
Depending on the isomeric state, photoswitches have the potential to replace transistors used in electronics. [37] Through the attachment of photoswitches onto the surfaces of various substrates, the work function can be changed. For example, the incorporation of diarylethenes as a self-assembled monolayer on a gold surface shows promise in optoelectronic devices. [38]
Diarylethenes form stable molecular conduction junctions when placed between graphene electrodes at low and room temperature and act as a photo-electrical switch. [39] By combining a photoswitch, containing various highest and lowest unoccupied molecular orbital levels in its open and closed geometrical conformation, into a film composed of either p- or n-doped semiconductors, charge transport can be controlled with light. [38] A photo-electric cell is connected to a circuit that measures how much electricity the cell generates. The circuit decides and gives the output, according to the setting of minimum and maximum lux level. [40]
Photoswitches have been used in the generation of three-dimensional animations and images. [41] The display utilizes a medium composed of a class of photoswitches (known as spirhodamines) and digital light processing technology to generate structured light in three dimensions. UV light and green light patterns are aimed at the dye solution, which initiates photoactivation and thus creates the 'on' voxel.
Energy storage
Due to one of the photoisomers being more stable than the other, isomerization from the stable to metastable isomer results in a conversion of light energy into free energy as a form of a chemical potential and has applications in storing solar energy. [42]
Merocyanine has been shown to shuttle protons across a polymeric membrane upon irradiation with light. When UV and visible light were irradiated upon opposites sides of the membrane, a storage potential and pH gradient were generated. [43]
Guest uptake and release
Incorporation of photoswitchable molecules into porous metal organic frameworks that can uptake of gaseous molecules like carbon dioxide as well as contribute to optoelectronics, nanomedicine, and better energy storage. By changing the chemical properties of the pores, adsorption and desorption of gases can be tuned for advancements in smart membrane materials. [43]
Nanoreactors and cell mimics
Incorporation of photoswitching molecules such as donor-acceptor Stenhouse adducts into polymersomes has been used to form nanoparticles which can selectively expose enzymes in response to light, allowing them to mimic some functions of cells. [44]
Liquid crystals
Chiral shape driven transformations in liquid crystal structures can be achieved through photoisomerization of bistable hydrazones to generate long term stable polymer shapes. [45] Light-gated optical windows that can change the absorbance properties can be made by chirally doping liquid crystals with hydrazone photoswitches or by kinetically trapping various cholesteric states as a function of the photostationary state. [46] Incorporation of photoswitches into nematic liquid crystals can change self-assembly, crystal packing, and the light reflecting properties of the supramolecular interactions. [47]
Optical storage
Diarylethene photoswitches have been promising for use in rewritable optical storage. Through irradiation of light, writing, erasing, and reading can parallel CD/ DVD storage with better performance. [48] Novel azo-carrying photoswitches are introduced as molecular hinges, [49] [50] which can be used in the design of molecular machines and optical devices. [51]
Photopharmacology
In the field of photopharmacology, photoswitches are being investigated as a means to control activity. By including a photoswitch in a drug, the drug assumes several biological active states. Light can be used to switch between these states, resulting in remote control of a drug's activity. Photoswitches have also been shown modulate surface energy properties which can control how the photoswitchable shell interacts with nanoparticles. [52] Pharmaceutical encapsulation and distribution at targeted locations with light has been demonstrated due to the unique change in properties and size of microencapsulated nanostructures with photochromic components. [53]
Self-healing materials
Photoswitches have been investigated for self-healable polymer materials. The first incorporates the phototunability of various functional groups so reactivity can be modulated in one of the isomeric forms, while the second strategy incorporates light-driven valence bond tautomerization. [43]
References
- ^ Aprahamian I (March 2020). "The Future of Molecular Machines". ACS Central Science. 6 (3): 347–358. doi: 10.1021/acscentsci.0c00064. PMC 7099591. PMID 32232135.
- ^ Kassem S, van Leeuwen T, Lubbe AS, Wilson MR, Feringa BL, Leigh DA (May 2017). "Artificial molecular motors". Chemical Society Reviews. 46 (9): 2592–2621. doi: 10.1039/C7CS00245A. PMID 28426052.
- ^ Cameron D, Eisler S (2018). "Photoswitchable double bonds: Synthetic strategies for tunability and versatility". Journal of Physical Organic Chemistry. 31 (10): e3858. doi: 10.1002/poc.3858. ISSN 1099-1395.
- ^ Goulet-Hanssens A, Eisenreich F, Hecht S (May 2020). "Enlightening Materials with Photoswitches". Advanced Materials. 32 (20): e1905966. Bibcode: 2020AdM....3205966G. doi: 10.1002/adma.201905966. PMID 31975456.
- ^ Qian H, Pramanik S, Aprahamian I (July 2017). "Photochromic Hydrazone Switches with Extremely Long Thermal Half-Lives". Journal of the American Chemical Society. 139 (27): 9140–9143. doi: 10.1021/jacs.7b04993. PMID 28644015.
- ^ Bandara HM, Burdette SC (March 2012). "Photoisomerization in different classes of azobenzene". Chemical Society Reviews. 41 (5): 1809–25. doi: 10.1039/C1CS15179G. PMID 22008710.
- ^ Kortekaas L, Browne WR (June 2019). "The evolution of spiropyran: fundamentals and progress of an extraordinarily versatile photochrome". Chemical Society Reviews. 48 (12): 3406–3424. doi: 10.1039/C9CS00203K. PMID 31150035.
- ^ Klajn R (January 2014). "Spiropyran-based dynamic materials". Chemical Society Reviews. 43 (1): 148–84. doi: 10.1039/C3CS60181A. PMID 23979515.
- ^ Pu SZ, Sun Q, Fan CB, Wang RJ, Liu G (2016-04-14). "Recent advances in diarylethene-based multi-responsive molecular switches". Journal of Materials Chemistry C. 4 (15): 3075–3093. doi: 10.1039/C6TC00110F. ISSN 2050-7534.
- ^ Berkovic G, Krongauz V, Weiss V (May 2000). "Spiropyrans and Spirooxazines for Memories and Switches". Chemical Reviews. 100 (5): 1741–1754. doi: 10.1021/cr9800715. PMID 11777418.
- ^ Yokoyama Y (May 2000). "Fulgides for Memories and Switches". Chemical Reviews. 100 (5): 1717–1740. doi: 10.1021/cr980070c. PMID 11777417.
- ^ Su X, Aprahamian I (March 2014). "Hydrazone-based switches, metallo-assemblies and sensors". Chemical Society Reviews. 43 (6): 1963–81. doi: 10.1039/C3CS60385G. PMID 24429467.
- ^ Orrego-Hernández J, Dreos A, Moth-Poulsen K (August 2020). "Engineering of Norbornadiene/Quadricyclane Photoswitches for Molecular Solar Thermal Energy Storage Applications". Accounts of Chemical Research. 53 (8): 1478–1487. doi: 10.1021/acs.accounts.0c00235. PMC 7467572. PMID 32662627.
- ^ Navrátil R, Wiedbrauk S, Jašík J, Dube H, Roithová J (March 2018). "Transforming hemithioindigo from a two-way to a one-way molecular photoswitch by isolation in the gas phase". Physical Chemistry Chemical Physics. 20 (10): 6868–6876. Bibcode: 2018PCCP...20.6868N. doi: 10.1039/C8CP00096D. PMID 29485646.
- ^ Polosukhina A, Litt J, Tochitsky I, Nemargut J, Sychev Y, De Kouchkovsky I, et al. (July 2012). "Photochemical restoration of visual responses in blind mice". Neuron. 75 (2): 271–82. doi: 10.1016/j.neuron.2012.05.022. PMC 3408583. PMID 22841312.
- ^ Lerch MM, Szymański W, Feringa BL (March 2018). "The (photo)chemistry of Stenhouse photoswitches: guiding principles and system design". Chemical Society Reviews. 47 (6): 1910–1937. doi: 10.1039/C7CS00772H. PMID 29468232.
- ^ Helmy, Sameh; Oh, Saemi; Leibfarth, Frank A.; Hawker, Craig J.; Read de Alaniz, Javier (2014-12-05). "Design and Synthesis of Donor–Acceptor Stenhouse Adducts: A Visible Light Photoswitch Derived from Furfural". The Journal of Organic Chemistry. 79 (23): 11316–11329. doi: 10.1021/jo502206g. ISSN 0022-3263. PMID 25390619.
- ^ Abourashed EA (2017-02-24). "Review of Stilbenes: Applications in Chemistry, Life Sciences and Materials Science". Journal of Natural Products. 80 (2): 577. doi: 10.1021/acs.jnatprod.7b00089. ISSN 0163-3864.
- ^ Roberts JD, Caserio MC (1977-05-15). Basic Principles of Organic Chemistry, second edition. Menlo Park, CA: W. A. Benjamin, Inc. ISBN 978-0-8053-8329-4.
- ^ Liu RS (July 2001). "Photoisomerization by hula-twist: a fundamental supramolecular photochemical reaction". Accounts of Chemical Research. 34 (7): 555–62. doi: 10.1021/ar000165c. PMID 11456473.
- ^ Liu RS, Hammond GS (October 2000). "The case of medium-dependent dual mechanisms for photoisomerization: one-bond-flip and hula-twist". Proceedings of the National Academy of Sciences of the United States of America. 97 (21): 11153–8. Bibcode: 2000PNAS...9711153L. doi: 10.1073/pnas.210323197. PMC 17169. PMID 11016972.
- ^ a b Stranius K, Börjesson K (January 2017). "Determining the Photoisomerization Quantum Yield of Photoswitchable Molecules in Solution and in the Solid State". Scientific Reports. 7 (1): 41145. Bibcode: 2017NatSR...741145S. doi: 10.1038/srep41145. PMC 5259717. PMID 28117426.
- ^ Gonzalez A, Kengmana ES, Fonseca MV, Han GG (June 2020). "Solid-state photoswitching molecules: structural design for isomerization in condensed phase". Materials Today Advances. 6: 100058. doi: 10.1016/j.mtadv.2020.100058.
- ^ Wegner HA (May 2012). "Azobenzenes in a new light-switching in vivo". Angewandte Chemie. 51 (20): 4787–8. doi: 10.1002/anie.201201336. PMID 22461191.
- ^ Dorel R, Feringa BL (June 2019). "Photoswitchable catalysis based on the isomerisation of double bonds". Chemical Communications. 55 (46): 6477–6486. doi: 10.1039/C9CC01891C. PMID 31099809.
- ^ "Photochemical Changes in Opsin". Chemistry LibreTexts. 2013-10-02. Retrieved 2021-02-24.
- ^ Ernst OP, Lodowski DT, Elstner M, Hegemann P, Brown LS, Kandori H (January 2014). "Microbial and animal rhodopsins: structures, functions, and molecular mechanisms". Chemical Reviews. 114 (1): 126–63. doi: 10.1021/cr4003769. PMC 3979449. PMID 24364740.
- ^ "Photochemical reaction | chemical reaction". Encyclopedia Britannica.
- ^ Inuzuka K, Becker RS (July 1968). "Mechanism of photoisomerization in the retinals and implications in rhodopsin". Nature. 219 (5152): 383–5. Bibcode: 1968Natur.219..383I. doi: 10.1038/219383a0. PMID 5667083. S2CID 4160990.
- ^ Kandori H (April 2020). "Biophysics of rhodopsins and optogenetics". Biophysical Reviews. 12 (2): 355–361. doi: 10.1007/s12551-020-00645-0. PMC 7242518. PMID 32065378.
- ^ Polosukhina A, Litt J, Tochitsky I, Nemargut J, Sychev Y, De Kouchkovsky I, et al. (July 2012). "Photochemical restoration of visual responses in blind mice". Neuron. 75 (2): 271–82. doi: 10.1016/j.neuron.2012.05.022. PMC 3408583. PMID 22841312.
- ^ Ratner M (February 2021). "Light-activated genetic therapy to treat blindness enters clinic". Nature Biotechnology. 39 (2): 126–127. doi: 10.1038/s41587-021-00823-9. PMID 33564161.
- ^ Szymański W, Beierle JM, Kistemaker HA, Velema WA, Feringa BL (August 2013). "Reversible photocontrol of biological systems by the incorporation of molecular photoswitches". Chemical Reviews. 113 (8): 6114–78. doi: 10.1021/cr300179f. PMID 23614556.
- ^ Putri RM, Zulfikri H, Fredy JW, Juan A, Tananchayakul P, Cornelissen JJ, et al. (July 2018). "Photoprogramming Allostery in Human Serum Albumin". Bioconjugate Chemistry. 29 (7): 2215–2224. doi: 10.1021/acs.bioconjchem.8b00184. PMC 6053643. PMID 29975051.
- ^ Kienzler MA, Reiner A, Trautman E, Yoo S, Trauner D, Isacoff EY (November 2013). "A red-shifted, fast-relaxing azobenzene photoswitch for visible light control of an ionotropic glutamate receptor". Journal of the American Chemical Society. 135 (47): 17683–6. doi: 10.1021/ja408104w. PMC 3990231. PMID 24171511.
- ^ Sinicropi A. "Biomimetic Photoswitches". Inorganica Chimica Acta. 470: 360–364. doi: 10.1016/j.ica.2017.08.041. ISSN 0020-1693.
- ^ "The future of electronics is photoswitches". BrandeisNOW.
- ^ a b Goulet-Hanssens A, Eisenreich F, Hecht S (May 2020). "Enlightening Materials with Photoswitches". Advanced Materials. 32 (20): e1905966. Bibcode: 2020AdM....3205966G. doi: 10.1002/adma.201905966. PMID 31975456.
- ^ Jia C, Migliore A, Xin N, Huang S, Wang J, Yang Q, et al. (June 2016). "Covalently bonded single-molecule junctions with stable and reversible photoswitched conductivity". Science. 352 (6292): 1443–5. Bibcode: 2016Sci...352.1443J. doi: 10.1126/science.aaf6298. PMID 27313042. S2CID 206649097.
- ^ Woodford, Chris (4 December 2009). "How do photoelectric cells work?". Explain that Stuff.
- ^ Patel SK, Cao J, Lippert AR (July 2017). "A volumetric three-dimensional digital light photoactivatable dye display". Nature Communications. 8: 15239. Bibcode: 2017NatCo...815239P. doi: 10.1038/ncomms15239. PMC 5508202. PMID 28695887.
- ^ Sun CL, Wang C, Boulatov R (2019). "Applications of Photoswitches in the Storage of Solar Energy". ChemPhotoChem. 3 (6): 268–283. doi: 10.1002/cptc.201900030. ISSN 2367-0932. S2CID 155439646.
- ^ a b c Goulet-Hanssens A, Eisenreich F, Hecht S (May 2020). "Enlightening Materials with Photoswitches". Advanced Materials. 32 (20): e1905966. Bibcode: 2020AdM....3205966G. doi: 10.1002/adma.201905966. PMID 31975456.
- ^ Rifaie-Graham, Omar; Yeow, Jonathan; Najer, Adrian; Wang, Richard; Sun, Rujie; Zhou, Kun; Dell, Tristan N.; Adrianus, Christopher; Thanapongpibul, Chalaisorn; Chami, Mohamed; Mann, Stephen; de Alaniz, Javier Read; Stevens, Molly M. (2022-11-07). "Photoswitchable gating of non-equilibrium enzymatic feedback in chemically communicating polymersome nanoreactors". Nature Chemistry. 15 (1): 110–118. doi: 10.1038/s41557-022-01062-4. ISSN 1755-4349. PMC 9836937. PMID 36344820.
- ^ Ryabchun A, Li Q, Lancia F, Aprahamian I, Katsonis N (January 2019). "Shape-Persistent Actuators from Hydrazone Photoswitches". Journal of the American Chemical Society. 141 (3): 1196–1200. doi: 10.1021/jacs.8b11558. PMC 6346373. PMID 30624915.
- ^ Moran MJ, Magrini M, Walba DM, Aprahamian I (October 2018). "Driving a Liquid Crystal Phase Transition Using a Photochromic Hydrazone". Journal of the American Chemical Society. 140 (42): 13623–13627. doi: 10.1021/jacs.8b09622. PMID 30293432. S2CID 207195468.
- ^ Zhang X, Koz B, Bisoyi HK, Wang H, Gutierrez-Cuevas KG, McConney ME, et al. (December 2020). "Electro- and Photo-Driven Orthogonal Switching of a Helical Superstructure Enabled by an Axially Chiral Molecular Switch". ACS Applied Materials & Interfaces. 12 (49): 55215–55222. doi: 10.1021/acsami.0c19527. PMID 33237715. S2CID 227174963.
- ^ Rosenbaum LC (November 7, 2018). "Organic Photochromic Compounds" (PDF). Universitat Konstanz.
- ^ Kazem-Rostami M, Moghanian A (2017). "Hünlich base derivatives as photo-responsive Λ-shaped hinges". Organic Chemistry Frontiers. 4 (2): 224–228. doi: 10.1039/C6QO00653A.
- ^ Norikane Y, Tamaoki N (July 2004). "Light-driven molecular hinge: a new molecular machine showing a light-intensity-dependent photoresponse that utilizes the trans-cis isomerization of azobenzene". Organic Letters. 6 (15): 2595–8. doi: 10.1021/ol049082c. PMID 15255699.
- ^ Kazem-Rostami M (5 December 2016). "Design and Synthesis of Ʌ-Shaped Photoswitchable Compounds Employing Tröger's Base Scaffold". Synthesis. 49 (6): 1214–1222. doi: 10.1055/s-0036-1588913. S2CID 99913657.
- ^ Velema WA, Szymanski W, Feringa BL (February 2014). "Photopharmacology: beyond proof of principle" (PDF). Journal of the American Chemical Society. 136 (6): 2178–91. doi: 10.1021/ja413063e. hdl: 11370/d6714f52-c2c8-4e48-b345-238e98bcc776. PMID 24456115. S2CID 197196311.
- ^ Guo X, Shao B, Zhou S, Aprahamian I, Chen Z (2020-03-18). "Visualizing intracellular particles and precise control of drug release using an emissive hydrazone photochrome". Chemical Science. 11 (11): 3016–3021. doi: 10.1039/C9SC05321B. ISSN 2041-6539. PMC 8157519. PMID 34122804.
A photoswitch is a type of molecule that can change its structural geometry and chemical properties upon irradiation with electromagnetic radiation. Although often used interchangeably with the term molecular machine, a switch does not perform work upon a change in its shape whereas a machine does. [1] However, photochromic compounds are the necessary building blocks for light driven molecular motors and machines. [2] Upon irradiation with light, photoisomerization about double bonds in the molecule can lead to changes in the cis- or trans- configuration. [3] These photochromic molecules are being considered for a range of applications.
Chemical structures and properties


A photochromic compound can change its configuration or structure upon irradiation with light. Several examples of photochromic compounds include: azobenzene, [6] spiropyran, [7] merocyanine, [8] diarylethene, [9] spirooxazine, [10] fulgide, [11] hydrazone, [12] nobormadiene, [13] thioindigo, [14] acrylamide-azobenzene-quaternary ammonia, [15] donor-acceptor Stenhouse adducts, [16] [17] stilbene, [18] etc.
Isomerization
Upon isomerization from the absorption of light, a π-to-π* or n-to-π* electronic transition can occur with the subsequent release of light ( fluorescence or phosphorescence) or heat when electrons transit from an excited state to a ground state. A photostationary state can be achieved when the irradiation of light no longer converts one form of an isomer into another; however, a mixture of cis- and trans- isomers will always exist with a higher percentage of one versus the other depending on the photoconditions. [19]
Mechanism
Although the mechanism for photoisomerization is still debated amongst most scientists, increasing evidence supports cis-/trans- isomerization of polyenes favoring the hula twist rather than the one-bond-flip. [20] The one-bond-flip isomerizes at the reactive double bond while the hula twist undergoes a conformational isomerization at the adjacent single bond. However, the interconversion of stereoisomers of stilbene proceeds via one-bond-flip. [21]
Quantum yield
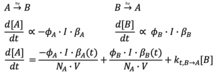
One of the most important properties of a photoswitch is its quantum yield which measures the effectiveness of absorbed light to induce photoisomerization. Quantum yield is modeled and calculated using Arrhenius kinetics. [22] Photoswitches can be in solution or in the solid state; however, switching in the solid state is more difficult to observe due to the lack of molecular freedom of motion, solid packing, and the fast thermal reversion to the ground state. [23] Through chemical modification, red shifting the wavelengths of absorption needed to cause isomerizaiton leads to low light induced switching which has applications in photopharmacology. [24]
Catalysis
When a photochromic compound is incorporated into a suitable catalytic molecule, photoswitchable catalysis can result from the reversible changes in geometric conformation upon irradiation with light. As one of the most widely studied photoswitches, azobenzene has been shown to be an effective switch for regulating catalytic activity due to its isomerization from the E to Z conformation with light, and its ability to thermally relax back to the E isomer in dark conditions. [25]
Biological

Rhodopsins
One of the more prevalent biological examples in the human body that undergoes structural changes upon light irradiation includes the class of membrane-bound photoreceptors, Rhodopsins. [27] These include the regulation of melanocytes, vision, the release of melatonin and the control of the circadian rhythm, etc. [28] Rhodopsins are highly efficient photochromic compounds that can undergo fast photoisomerization and are associated with various retinal proteins [29] along with light-gated channels and pumps in microbes. [30]
Research
Advances in vision restoration with photochromic compounds has been investigated. Fast isomerization allows retinal cells to turn on when activated by light and advances in acrylamide-azobenzene-quaternary ammonia have shown restoration of visual responses in blind mice. [31] Companies involved in this area include Novartis, Vedere, Allergan, and Nanoscope Therapeutics. [32]
Through the incorporation of photoswitches into biological molecules, biological processes can be regulated through controlled irradiation with light. This includes photocontrol of peptide conformation and activity, transcription and translation of DNA and RNA, regulation of enzymatic activity, and photoregulated ion channels. [33] For example, optical control of ligand binding in human serum albumin has been demonstrated to influence its allosteric binding properties. [34] Also, red-shifted azobenzenes have been used to control ionotropic glutamate receptors. [35]
Potential applications
Photoswitches are studied in biology, materials chemistry, and physics and have a wide variety of potential applications, especially in the framework of nanotechnology. [36]
Electronics
Depending on the isomeric state, photoswitches have the potential to replace transistors used in electronics. [37] Through the attachment of photoswitches onto the surfaces of various substrates, the work function can be changed. For example, the incorporation of diarylethenes as a self-assembled monolayer on a gold surface shows promise in optoelectronic devices. [38]
Diarylethenes form stable molecular conduction junctions when placed between graphene electrodes at low and room temperature and act as a photo-electrical switch. [39] By combining a photoswitch, containing various highest and lowest unoccupied molecular orbital levels in its open and closed geometrical conformation, into a film composed of either p- or n-doped semiconductors, charge transport can be controlled with light. [38] A photo-electric cell is connected to a circuit that measures how much electricity the cell generates. The circuit decides and gives the output, according to the setting of minimum and maximum lux level. [40]
Photoswitches have been used in the generation of three-dimensional animations and images. [41] The display utilizes a medium composed of a class of photoswitches (known as spirhodamines) and digital light processing technology to generate structured light in three dimensions. UV light and green light patterns are aimed at the dye solution, which initiates photoactivation and thus creates the 'on' voxel.
Energy storage
Due to one of the photoisomers being more stable than the other, isomerization from the stable to metastable isomer results in a conversion of light energy into free energy as a form of a chemical potential and has applications in storing solar energy. [42]
Merocyanine has been shown to shuttle protons across a polymeric membrane upon irradiation with light. When UV and visible light were irradiated upon opposites sides of the membrane, a storage potential and pH gradient were generated. [43]
Guest uptake and release
Incorporation of photoswitchable molecules into porous metal organic frameworks that can uptake of gaseous molecules like carbon dioxide as well as contribute to optoelectronics, nanomedicine, and better energy storage. By changing the chemical properties of the pores, adsorption and desorption of gases can be tuned for advancements in smart membrane materials. [43]
Nanoreactors and cell mimics
Incorporation of photoswitching molecules such as donor-acceptor Stenhouse adducts into polymersomes has been used to form nanoparticles which can selectively expose enzymes in response to light, allowing them to mimic some functions of cells. [44]
Liquid crystals
Chiral shape driven transformations in liquid crystal structures can be achieved through photoisomerization of bistable hydrazones to generate long term stable polymer shapes. [45] Light-gated optical windows that can change the absorbance properties can be made by chirally doping liquid crystals with hydrazone photoswitches or by kinetically trapping various cholesteric states as a function of the photostationary state. [46] Incorporation of photoswitches into nematic liquid crystals can change self-assembly, crystal packing, and the light reflecting properties of the supramolecular interactions. [47]
Optical storage
Diarylethene photoswitches have been promising for use in rewritable optical storage. Through irradiation of light, writing, erasing, and reading can parallel CD/ DVD storage with better performance. [48] Novel azo-carrying photoswitches are introduced as molecular hinges, [49] [50] which can be used in the design of molecular machines and optical devices. [51]
Photopharmacology
In the field of photopharmacology, photoswitches are being investigated as a means to control activity. By including a photoswitch in a drug, the drug assumes several biological active states. Light can be used to switch between these states, resulting in remote control of a drug's activity. Photoswitches have also been shown modulate surface energy properties which can control how the photoswitchable shell interacts with nanoparticles. [52] Pharmaceutical encapsulation and distribution at targeted locations with light has been demonstrated due to the unique change in properties and size of microencapsulated nanostructures with photochromic components. [53]
Self-healing materials
Photoswitches have been investigated for self-healable polymer materials. The first incorporates the phototunability of various functional groups so reactivity can be modulated in one of the isomeric forms, while the second strategy incorporates light-driven valence bond tautomerization. [43]
References
- ^ Aprahamian I (March 2020). "The Future of Molecular Machines". ACS Central Science. 6 (3): 347–358. doi: 10.1021/acscentsci.0c00064. PMC 7099591. PMID 32232135.
- ^ Kassem S, van Leeuwen T, Lubbe AS, Wilson MR, Feringa BL, Leigh DA (May 2017). "Artificial molecular motors". Chemical Society Reviews. 46 (9): 2592–2621. doi: 10.1039/C7CS00245A. PMID 28426052.
- ^ Cameron D, Eisler S (2018). "Photoswitchable double bonds: Synthetic strategies for tunability and versatility". Journal of Physical Organic Chemistry. 31 (10): e3858. doi: 10.1002/poc.3858. ISSN 1099-1395.
- ^ Goulet-Hanssens A, Eisenreich F, Hecht S (May 2020). "Enlightening Materials with Photoswitches". Advanced Materials. 32 (20): e1905966. Bibcode: 2020AdM....3205966G. doi: 10.1002/adma.201905966. PMID 31975456.
- ^ Qian H, Pramanik S, Aprahamian I (July 2017). "Photochromic Hydrazone Switches with Extremely Long Thermal Half-Lives". Journal of the American Chemical Society. 139 (27): 9140–9143. doi: 10.1021/jacs.7b04993. PMID 28644015.
- ^ Bandara HM, Burdette SC (March 2012). "Photoisomerization in different classes of azobenzene". Chemical Society Reviews. 41 (5): 1809–25. doi: 10.1039/C1CS15179G. PMID 22008710.
- ^ Kortekaas L, Browne WR (June 2019). "The evolution of spiropyran: fundamentals and progress of an extraordinarily versatile photochrome". Chemical Society Reviews. 48 (12): 3406–3424. doi: 10.1039/C9CS00203K. PMID 31150035.
- ^ Klajn R (January 2014). "Spiropyran-based dynamic materials". Chemical Society Reviews. 43 (1): 148–84. doi: 10.1039/C3CS60181A. PMID 23979515.
- ^ Pu SZ, Sun Q, Fan CB, Wang RJ, Liu G (2016-04-14). "Recent advances in diarylethene-based multi-responsive molecular switches". Journal of Materials Chemistry C. 4 (15): 3075–3093. doi: 10.1039/C6TC00110F. ISSN 2050-7534.
- ^ Berkovic G, Krongauz V, Weiss V (May 2000). "Spiropyrans and Spirooxazines for Memories and Switches". Chemical Reviews. 100 (5): 1741–1754. doi: 10.1021/cr9800715. PMID 11777418.
- ^ Yokoyama Y (May 2000). "Fulgides for Memories and Switches". Chemical Reviews. 100 (5): 1717–1740. doi: 10.1021/cr980070c. PMID 11777417.
- ^ Su X, Aprahamian I (March 2014). "Hydrazone-based switches, metallo-assemblies and sensors". Chemical Society Reviews. 43 (6): 1963–81. doi: 10.1039/C3CS60385G. PMID 24429467.
- ^ Orrego-Hernández J, Dreos A, Moth-Poulsen K (August 2020). "Engineering of Norbornadiene/Quadricyclane Photoswitches for Molecular Solar Thermal Energy Storage Applications". Accounts of Chemical Research. 53 (8): 1478–1487. doi: 10.1021/acs.accounts.0c00235. PMC 7467572. PMID 32662627.
- ^ Navrátil R, Wiedbrauk S, Jašík J, Dube H, Roithová J (March 2018). "Transforming hemithioindigo from a two-way to a one-way molecular photoswitch by isolation in the gas phase". Physical Chemistry Chemical Physics. 20 (10): 6868–6876. Bibcode: 2018PCCP...20.6868N. doi: 10.1039/C8CP00096D. PMID 29485646.
- ^ Polosukhina A, Litt J, Tochitsky I, Nemargut J, Sychev Y, De Kouchkovsky I, et al. (July 2012). "Photochemical restoration of visual responses in blind mice". Neuron. 75 (2): 271–82. doi: 10.1016/j.neuron.2012.05.022. PMC 3408583. PMID 22841312.
- ^ Lerch MM, Szymański W, Feringa BL (March 2018). "The (photo)chemistry of Stenhouse photoswitches: guiding principles and system design". Chemical Society Reviews. 47 (6): 1910–1937. doi: 10.1039/C7CS00772H. PMID 29468232.
- ^ Helmy, Sameh; Oh, Saemi; Leibfarth, Frank A.; Hawker, Craig J.; Read de Alaniz, Javier (2014-12-05). "Design and Synthesis of Donor–Acceptor Stenhouse Adducts: A Visible Light Photoswitch Derived from Furfural". The Journal of Organic Chemistry. 79 (23): 11316–11329. doi: 10.1021/jo502206g. ISSN 0022-3263. PMID 25390619.
- ^ Abourashed EA (2017-02-24). "Review of Stilbenes: Applications in Chemistry, Life Sciences and Materials Science". Journal of Natural Products. 80 (2): 577. doi: 10.1021/acs.jnatprod.7b00089. ISSN 0163-3864.
- ^ Roberts JD, Caserio MC (1977-05-15). Basic Principles of Organic Chemistry, second edition. Menlo Park, CA: W. A. Benjamin, Inc. ISBN 978-0-8053-8329-4.
- ^ Liu RS (July 2001). "Photoisomerization by hula-twist: a fundamental supramolecular photochemical reaction". Accounts of Chemical Research. 34 (7): 555–62. doi: 10.1021/ar000165c. PMID 11456473.
- ^ Liu RS, Hammond GS (October 2000). "The case of medium-dependent dual mechanisms for photoisomerization: one-bond-flip and hula-twist". Proceedings of the National Academy of Sciences of the United States of America. 97 (21): 11153–8. Bibcode: 2000PNAS...9711153L. doi: 10.1073/pnas.210323197. PMC 17169. PMID 11016972.
- ^ a b Stranius K, Börjesson K (January 2017). "Determining the Photoisomerization Quantum Yield of Photoswitchable Molecules in Solution and in the Solid State". Scientific Reports. 7 (1): 41145. Bibcode: 2017NatSR...741145S. doi: 10.1038/srep41145. PMC 5259717. PMID 28117426.
- ^ Gonzalez A, Kengmana ES, Fonseca MV, Han GG (June 2020). "Solid-state photoswitching molecules: structural design for isomerization in condensed phase". Materials Today Advances. 6: 100058. doi: 10.1016/j.mtadv.2020.100058.
- ^ Wegner HA (May 2012). "Azobenzenes in a new light-switching in vivo". Angewandte Chemie. 51 (20): 4787–8. doi: 10.1002/anie.201201336. PMID 22461191.
- ^ Dorel R, Feringa BL (June 2019). "Photoswitchable catalysis based on the isomerisation of double bonds". Chemical Communications. 55 (46): 6477–6486. doi: 10.1039/C9CC01891C. PMID 31099809.
- ^ "Photochemical Changes in Opsin". Chemistry LibreTexts. 2013-10-02. Retrieved 2021-02-24.
- ^ Ernst OP, Lodowski DT, Elstner M, Hegemann P, Brown LS, Kandori H (January 2014). "Microbial and animal rhodopsins: structures, functions, and molecular mechanisms". Chemical Reviews. 114 (1): 126–63. doi: 10.1021/cr4003769. PMC 3979449. PMID 24364740.
- ^ "Photochemical reaction | chemical reaction". Encyclopedia Britannica.
- ^ Inuzuka K, Becker RS (July 1968). "Mechanism of photoisomerization in the retinals and implications in rhodopsin". Nature. 219 (5152): 383–5. Bibcode: 1968Natur.219..383I. doi: 10.1038/219383a0. PMID 5667083. S2CID 4160990.
- ^ Kandori H (April 2020). "Biophysics of rhodopsins and optogenetics". Biophysical Reviews. 12 (2): 355–361. doi: 10.1007/s12551-020-00645-0. PMC 7242518. PMID 32065378.
- ^ Polosukhina A, Litt J, Tochitsky I, Nemargut J, Sychev Y, De Kouchkovsky I, et al. (July 2012). "Photochemical restoration of visual responses in blind mice". Neuron. 75 (2): 271–82. doi: 10.1016/j.neuron.2012.05.022. PMC 3408583. PMID 22841312.
- ^ Ratner M (February 2021). "Light-activated genetic therapy to treat blindness enters clinic". Nature Biotechnology. 39 (2): 126–127. doi: 10.1038/s41587-021-00823-9. PMID 33564161.
- ^ Szymański W, Beierle JM, Kistemaker HA, Velema WA, Feringa BL (August 2013). "Reversible photocontrol of biological systems by the incorporation of molecular photoswitches". Chemical Reviews. 113 (8): 6114–78. doi: 10.1021/cr300179f. PMID 23614556.
- ^ Putri RM, Zulfikri H, Fredy JW, Juan A, Tananchayakul P, Cornelissen JJ, et al. (July 2018). "Photoprogramming Allostery in Human Serum Albumin". Bioconjugate Chemistry. 29 (7): 2215–2224. doi: 10.1021/acs.bioconjchem.8b00184. PMC 6053643. PMID 29975051.
- ^ Kienzler MA, Reiner A, Trautman E, Yoo S, Trauner D, Isacoff EY (November 2013). "A red-shifted, fast-relaxing azobenzene photoswitch for visible light control of an ionotropic glutamate receptor". Journal of the American Chemical Society. 135 (47): 17683–6. doi: 10.1021/ja408104w. PMC 3990231. PMID 24171511.
- ^ Sinicropi A. "Biomimetic Photoswitches". Inorganica Chimica Acta. 470: 360–364. doi: 10.1016/j.ica.2017.08.041. ISSN 0020-1693.
- ^ "The future of electronics is photoswitches". BrandeisNOW.
- ^ a b Goulet-Hanssens A, Eisenreich F, Hecht S (May 2020). "Enlightening Materials with Photoswitches". Advanced Materials. 32 (20): e1905966. Bibcode: 2020AdM....3205966G. doi: 10.1002/adma.201905966. PMID 31975456.
- ^ Jia C, Migliore A, Xin N, Huang S, Wang J, Yang Q, et al. (June 2016). "Covalently bonded single-molecule junctions with stable and reversible photoswitched conductivity". Science. 352 (6292): 1443–5. Bibcode: 2016Sci...352.1443J. doi: 10.1126/science.aaf6298. PMID 27313042. S2CID 206649097.
- ^ Woodford, Chris (4 December 2009). "How do photoelectric cells work?". Explain that Stuff.
- ^ Patel SK, Cao J, Lippert AR (July 2017). "A volumetric three-dimensional digital light photoactivatable dye display". Nature Communications. 8: 15239. Bibcode: 2017NatCo...815239P. doi: 10.1038/ncomms15239. PMC 5508202. PMID 28695887.
- ^ Sun CL, Wang C, Boulatov R (2019). "Applications of Photoswitches in the Storage of Solar Energy". ChemPhotoChem. 3 (6): 268–283. doi: 10.1002/cptc.201900030. ISSN 2367-0932. S2CID 155439646.
- ^ a b c Goulet-Hanssens A, Eisenreich F, Hecht S (May 2020). "Enlightening Materials with Photoswitches". Advanced Materials. 32 (20): e1905966. Bibcode: 2020AdM....3205966G. doi: 10.1002/adma.201905966. PMID 31975456.
- ^ Rifaie-Graham, Omar; Yeow, Jonathan; Najer, Adrian; Wang, Richard; Sun, Rujie; Zhou, Kun; Dell, Tristan N.; Adrianus, Christopher; Thanapongpibul, Chalaisorn; Chami, Mohamed; Mann, Stephen; de Alaniz, Javier Read; Stevens, Molly M. (2022-11-07). "Photoswitchable gating of non-equilibrium enzymatic feedback in chemically communicating polymersome nanoreactors". Nature Chemistry. 15 (1): 110–118. doi: 10.1038/s41557-022-01062-4. ISSN 1755-4349. PMC 9836937. PMID 36344820.
- ^ Ryabchun A, Li Q, Lancia F, Aprahamian I, Katsonis N (January 2019). "Shape-Persistent Actuators from Hydrazone Photoswitches". Journal of the American Chemical Society. 141 (3): 1196–1200. doi: 10.1021/jacs.8b11558. PMC 6346373. PMID 30624915.
- ^ Moran MJ, Magrini M, Walba DM, Aprahamian I (October 2018). "Driving a Liquid Crystal Phase Transition Using a Photochromic Hydrazone". Journal of the American Chemical Society. 140 (42): 13623–13627. doi: 10.1021/jacs.8b09622. PMID 30293432. S2CID 207195468.
- ^ Zhang X, Koz B, Bisoyi HK, Wang H, Gutierrez-Cuevas KG, McConney ME, et al. (December 2020). "Electro- and Photo-Driven Orthogonal Switching of a Helical Superstructure Enabled by an Axially Chiral Molecular Switch". ACS Applied Materials & Interfaces. 12 (49): 55215–55222. doi: 10.1021/acsami.0c19527. PMID 33237715. S2CID 227174963.
- ^ Rosenbaum LC (November 7, 2018). "Organic Photochromic Compounds" (PDF). Universitat Konstanz.
- ^ Kazem-Rostami M, Moghanian A (2017). "Hünlich base derivatives as photo-responsive Λ-shaped hinges". Organic Chemistry Frontiers. 4 (2): 224–228. doi: 10.1039/C6QO00653A.
- ^ Norikane Y, Tamaoki N (July 2004). "Light-driven molecular hinge: a new molecular machine showing a light-intensity-dependent photoresponse that utilizes the trans-cis isomerization of azobenzene". Organic Letters. 6 (15): 2595–8. doi: 10.1021/ol049082c. PMID 15255699.
- ^ Kazem-Rostami M (5 December 2016). "Design and Synthesis of Ʌ-Shaped Photoswitchable Compounds Employing Tröger's Base Scaffold". Synthesis. 49 (6): 1214–1222. doi: 10.1055/s-0036-1588913. S2CID 99913657.
- ^ Velema WA, Szymanski W, Feringa BL (February 2014). "Photopharmacology: beyond proof of principle" (PDF). Journal of the American Chemical Society. 136 (6): 2178–91. doi: 10.1021/ja413063e. hdl: 11370/d6714f52-c2c8-4e48-b345-238e98bcc776. PMID 24456115. S2CID 197196311.
- ^ Guo X, Shao B, Zhou S, Aprahamian I, Chen Z (2020-03-18). "Visualizing intracellular particles and precise control of drug release using an emissive hydrazone photochrome". Chemical Science. 11 (11): 3016–3021. doi: 10.1039/C9SC05321B. ISSN 2041-6539. PMC 8157519. PMID 34122804.