| |
| Names | |
|---|---|
|
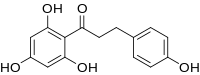
Preferred IUPAC name
3-(4-Hydroxyphenyl)-1-(2,4,6-trihydroxyphenyl)propan-1-one | |
| Other names
Dihydronaringenin
Phloretol | |
| Identifiers | |
3D model (
JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.000.444 |
| KEGG | |
PubChem
CID
|
|
| UNII | |
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C15H14O5 | |
| Molar mass | 274.272 g·mol−1 |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Phloretin is a dihydrochalcone, a type of natural phenol. It can be found in apple tree leaves [1] and the Manchurian apricot. [2]
Metabolism
In rats, ingested phlorizin is converted into phloretin by hydrolytic enzymes in the small intestine. [3] [4] Phloretin hydrolase hydrolyses phloretin into phloretic acid and phloroglucinol.
Pharmacological research
In an animal model, phloretin inhibited active transport of glucose into cells by SGLT1 and SGLT2, though the inhibition is weaker than by its glycoside phlorizin. [5] An important effect of this is the inhibition of glucose absorption by the small intestine [4] and the inhibition of renal glucose reabsorption. [3] Phloretin also inhibits a variety of urea transporters. [6] [7] It induces urea loss and diuresis when coupled with high protein diets. Phloretin has been found to inhibit weight gain and improve metabolic homeostasis in mice fed with high-fat diet. [8] Phloretin inhibits aquaporin 9 (AQP9) on mouse hepatocytes. [9]
Nanoparticle Synthesis
Phloretin functionalized gold-nanoparticles (Pht-GNPs) were synthesized using a single-step synthesis method and tested for its anticancer activity. Pht-GNPs showed significant cancer cell toxicities compared to free phloretin. [10]
Glycosides
- Phlorizin is the 2'- glucoside of phloretin
- Naringin dihydrochalcone is a diglycoside of phloretin
See also
References
- ^ Picinelli A.; Dapena E.; Mangas J. J. (1995). "Polyphenolic pattern in apple tree leaves in relation to scab resistance. A preliminary study". Journal of Agricultural and Food Chemistry. 43 (8): 2273–2278. doi: 10.1021/jf00056a057.
- ^ "Manchurian Apricot (Prunus armeniaca var. mandshurica)" (PDF). North Dakota State University. Retrieved January 30, 2014.
- ^ a b Idris, I.; Donnelly, R. (2009). "Sodium-glucose co-transporter-2 inhibitors: An emerging new class of oral antidiabetic drug". Diabetes, Obesity and Metabolism. 11 (2): 79–88. doi: 10.1111/j.1463-1326.2008.00982.x. PMID 19125776.
- ^ a b Crespy, V.; Aprikian, O.; Morand, C.; Besson, C.; Manach, C.; Demigné, C.; Rémésy, C. (2001). "Bioavailability of phloretin and phloridzin in rats". The Journal of Nutrition. 131 (12): 3227–3230. doi: 10.1093/jn/131.12.3227. PMID 11739871.
- ^ Chan, Stephen S.; William D. Lotspeich (1962-12-01). "Comparative effects of phlorizin and phloretin on glucose transport in the cat kidney". American Journal of Physiology. Legacy Content. 203 (6): 975–979. doi: 10.1152/ajplegacy.1962.203.6.975. ISSN 0002-9513. PMID 14019989. Retrieved 2012-10-21.
- ^ Fenton, Robert A.; Chung-Lin Chou; Gavin S. Stewart; Craig P. Smith; Mark A. Knepper (2004-05-11). "Urinary concentrating defect in mice with selective deletion of phloretin-sensitive urea transporters in the renal collecting duct". Proceedings of the National Academy of Sciences of the United States of America. 101 (19): 7469–7474. Bibcode: 2004PNAS..101.7469F. doi: 10.1073/pnas.0401704101. ISSN 0027-8424. PMC 409942. PMID 15123796.
- ^ Shayakul, Chairat; Hiroyasu Tsukaguchi; Urs V. Berger; Matthias A. Hediger (2001-03-01). "Molecular characterization of a novel urea transporter from kidney inner medullary collecting ducts". American Journal of Physiology. Renal Physiology. 280 (3): F487–F494. doi: 10.1152/ajprenal.2001.280.3.f487. ISSN 1931-857X. PMID 11181411. S2CID 22143248. Archived from the original on 2016-03-04. Retrieved 2012-10-21.
- ^ Alsanea, Sary; Gao, Mingming; Liu, Dexi (May 2017). "Phloretin Prevents High-Fat Diet-Induced Obesity and Improves Metabolic Homeostasis". The AAPS Journal. 19 (3): 797–805. doi: 10.1208/s12248-017-0053-0. ISSN 1550-7416. PMID 28197827. S2CID 3638970.
- ^ Fenton, Robert A.; Chou, Chung-Lin; Stewart, Gavin S.; Smith, Craig P.; Knepper, Mark A. (2004-05-11). "Urinary concentrating defect in mice with selective deletion of phloretin-sensitive urea transporters in the renal collecting duct". Proceedings of the National Academy of Sciences of the United States of America. 101 (19): 7469–7474. Bibcode: 2004PNAS..101.7469F. doi: 10.1073/pnas.0401704101. ISSN 0027-8424. PMC 409942. PMID 15123796.
- ^ Payne NJ, Badwaik VD, Waghwani HK, Moolani HV, Tockstein S, Thompson DH, Dakshinamurthy R (March 2018). "Development of dihydrochalcone-functionalized gold nanoparticles for augmented antineoplastic activity". International Journal of Nanomedicine. 13: 1917–1926. doi: 10.2147/IJN.S143506. PMC 5880570. PMID 29636609.

| |
| Names | |
|---|---|
|
Preferred IUPAC name
3-(4-Hydroxyphenyl)-1-(2,4,6-trihydroxyphenyl)propan-1-one | |
| Other names
Dihydronaringenin
Phloretol | |
| Identifiers | |
3D model (
JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.000.444 |
| KEGG | |
PubChem
CID
|
|
| UNII | |
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C15H14O5 | |
| Molar mass | 274.272 g·mol−1 |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Phloretin is a dihydrochalcone, a type of natural phenol. It can be found in apple tree leaves [1] and the Manchurian apricot. [2]
Metabolism
In rats, ingested phlorizin is converted into phloretin by hydrolytic enzymes in the small intestine. [3] [4] Phloretin hydrolase hydrolyses phloretin into phloretic acid and phloroglucinol.
Pharmacological research
In an animal model, phloretin inhibited active transport of glucose into cells by SGLT1 and SGLT2, though the inhibition is weaker than by its glycoside phlorizin. [5] An important effect of this is the inhibition of glucose absorption by the small intestine [4] and the inhibition of renal glucose reabsorption. [3] Phloretin also inhibits a variety of urea transporters. [6] [7] It induces urea loss and diuresis when coupled with high protein diets. Phloretin has been found to inhibit weight gain and improve metabolic homeostasis in mice fed with high-fat diet. [8] Phloretin inhibits aquaporin 9 (AQP9) on mouse hepatocytes. [9]
Nanoparticle Synthesis
Phloretin functionalized gold-nanoparticles (Pht-GNPs) were synthesized using a single-step synthesis method and tested for its anticancer activity. Pht-GNPs showed significant cancer cell toxicities compared to free phloretin. [10]
Glycosides
- Phlorizin is the 2'- glucoside of phloretin
- Naringin dihydrochalcone is a diglycoside of phloretin
See also
References
- ^ Picinelli A.; Dapena E.; Mangas J. J. (1995). "Polyphenolic pattern in apple tree leaves in relation to scab resistance. A preliminary study". Journal of Agricultural and Food Chemistry. 43 (8): 2273–2278. doi: 10.1021/jf00056a057.
- ^ "Manchurian Apricot (Prunus armeniaca var. mandshurica)" (PDF). North Dakota State University. Retrieved January 30, 2014.
- ^ a b Idris, I.; Donnelly, R. (2009). "Sodium-glucose co-transporter-2 inhibitors: An emerging new class of oral antidiabetic drug". Diabetes, Obesity and Metabolism. 11 (2): 79–88. doi: 10.1111/j.1463-1326.2008.00982.x. PMID 19125776.
- ^ a b Crespy, V.; Aprikian, O.; Morand, C.; Besson, C.; Manach, C.; Demigné, C.; Rémésy, C. (2001). "Bioavailability of phloretin and phloridzin in rats". The Journal of Nutrition. 131 (12): 3227–3230. doi: 10.1093/jn/131.12.3227. PMID 11739871.
- ^ Chan, Stephen S.; William D. Lotspeich (1962-12-01). "Comparative effects of phlorizin and phloretin on glucose transport in the cat kidney". American Journal of Physiology. Legacy Content. 203 (6): 975–979. doi: 10.1152/ajplegacy.1962.203.6.975. ISSN 0002-9513. PMID 14019989. Retrieved 2012-10-21.
- ^ Fenton, Robert A.; Chung-Lin Chou; Gavin S. Stewart; Craig P. Smith; Mark A. Knepper (2004-05-11). "Urinary concentrating defect in mice with selective deletion of phloretin-sensitive urea transporters in the renal collecting duct". Proceedings of the National Academy of Sciences of the United States of America. 101 (19): 7469–7474. Bibcode: 2004PNAS..101.7469F. doi: 10.1073/pnas.0401704101. ISSN 0027-8424. PMC 409942. PMID 15123796.
- ^ Shayakul, Chairat; Hiroyasu Tsukaguchi; Urs V. Berger; Matthias A. Hediger (2001-03-01). "Molecular characterization of a novel urea transporter from kidney inner medullary collecting ducts". American Journal of Physiology. Renal Physiology. 280 (3): F487–F494. doi: 10.1152/ajprenal.2001.280.3.f487. ISSN 1931-857X. PMID 11181411. S2CID 22143248. Archived from the original on 2016-03-04. Retrieved 2012-10-21.
- ^ Alsanea, Sary; Gao, Mingming; Liu, Dexi (May 2017). "Phloretin Prevents High-Fat Diet-Induced Obesity and Improves Metabolic Homeostasis". The AAPS Journal. 19 (3): 797–805. doi: 10.1208/s12248-017-0053-0. ISSN 1550-7416. PMID 28197827. S2CID 3638970.
- ^ Fenton, Robert A.; Chou, Chung-Lin; Stewart, Gavin S.; Smith, Craig P.; Knepper, Mark A. (2004-05-11). "Urinary concentrating defect in mice with selective deletion of phloretin-sensitive urea transporters in the renal collecting duct". Proceedings of the National Academy of Sciences of the United States of America. 101 (19): 7469–7474. Bibcode: 2004PNAS..101.7469F. doi: 10.1073/pnas.0401704101. ISSN 0027-8424. PMC 409942. PMID 15123796.
- ^ Payne NJ, Badwaik VD, Waghwani HK, Moolani HV, Tockstein S, Thompson DH, Dakshinamurthy R (March 2018). "Development of dihydrochalcone-functionalized gold nanoparticles for augmented antineoplastic activity". International Journal of Nanomedicine. 13: 1917–1926. doi: 10.2147/IJN.S143506. PMC 5880570. PMID 29636609.