
| |
| Names | |
|---|---|
|
IUPAC name
3,4′,5,7-Tetrahydroxyflavylium
| |
|
Systematic IUPAC name
3,5,7-Trihydroxy-2-(4-hydroxyphenyl)-1λ4-benzopyran-4-ylium | |
| Identifiers | |
3D model (
JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem
CID
|
|
| UNII | |
| |
| |
| Properties | |
| C15H11O5+ | |
| Molar mass | 271.24 g/mol |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Pelargonidin is an anthocyanidin, a type of plant pigment producing a characteristic orange color used in food and industrial dyes. [1]
Natural occurrences
Presence in flowers
Pelargonidin can be found in red geraniums (Geraniaceae). It is the predominant pigment causing the red coloration in the spathes of Philodendron (Araceae). The orange-coloured flowers of blue pimpernel ( Anagallis monelli, Myrsinaceae) have a higher concentration of pelargonidin pigment. Red and Pink Roses (Rosa) obtain their color from this phytochemical. [2]
Presence in food
Pelargonidin can be found in berries such as ripe raspberries and strawberries, as well as blueberries, blackberries, cranberries but also in saskatoon berries [3] and chokeberries. It is also found in plums and pomegranates. Pelargonidin gives red radishes their color. [4]
It is present in large amounts in kidney beans. [5]
Glycosides
In many plant systems, Pelargonidin can be added to a glucose molecule to form Pelargonidin 3-glucoside (callistephin). This is done by the 3GT, anthocyanin 3-O-glucosyltransferase gene. [6]
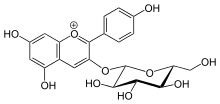
However this glucosidation reduces its antioxidant activity, [7] and changes the wavelength of max light absorbance from 520 nm to 516 nm. [8]
Acylated pelargonidin glycosides can be found in red-purple flowers of Ipomoea purpurea. [9]
See also
References
- ^ US granted 6,767,999, Smirnov, Vitaly; Sidorov, Viktor; Smirnova, Valentina, "Anthocyantin coloring agent and method for the production thereof from organic matter", published Nov 01, 2001, issued July 27, 2004
- ^ Huihua, Wan; Chao, Yu; Yu, Han; Xuelian, Guo (2019). "Determination of Flavonoids and Carotenoids and Their Contributions to Various Colors of Rose Cultivars (Rosa spp.)". Frontiers in Plant Science. 10: 123. doi: 10.3389/fpls.2019.00123. PMC 6379320. PMID 30809238.
- ^ Mazza, G. (2005). "Compositional and Functional Properties of Saskatoon Berry and Blueberry". International Journal of Fruit Science. 5 (3): 101–120. doi: 10.1300/J492v05n03_10. S2CID 85691882.
- ^ Takeshi Nishio (4 October 2017). Takeshi Nishio, Hiroyasu Kitashiba (ed.). The Radish Genome. Springer. p. 4. ISBN 978-3-319-59253-4.
- ^ Lin, Long-Ze; Harnly, James M.; Pastor-Corrales, Marcial S.; Luthria, Devanand L. (2008). "The polyphenolic profiles of common bean (Phaseolus vulgaris L.)". Food Chemistry. 107 (1): 399–410. doi: 10.1016/j.foodchem.2007.08.038. PMC 4276374. PMID 25544796.
- ^ Levisson, Mark; Patinios, Constantinios; Hein, Sascha; de Groot, Phillip A. (2018). "Engineering de novo anthocyanin production in Saccharomyces cerevisiae". Microbial Cell Factories. 17 (103): 103. doi: 10.1186/s12934-018-0951-6. PMC 6029064. PMID 29970082.
- ^ Li, Wenfeng; Gu, Mengyuan; Gong, Pengling; Wang, Jinxia (2021). "Glycosides changed the stability and antioxidant activity of pelargonidin". Lebensmittel-Wissenschaft & Technologie. 147 (3): 111581. doi: 10.1016/j.lwt.2021.111581. S2CID 235531625.
- ^ Gould, Kevin S. (2009). Anthocyanidins: Biosynthesis, Functions, and Applications. New York: Springer. p. 286. ISBN 978-0-387-77334-6.
- ^ Saito, N; Tatsuzawa, F; Yokoi, M; Kasahara, K; Iida, S; Shigihara, A; Honda, T (1996). "Acylated pelargonidin glycosides in red-purple flowers of Ipomoea purpurea". Phytochemistry. 43 (6): 1365–70. doi: 10.1016/s0031-9422(96)00501-8. PMID 8987912.
External links

| |
| Names | |
|---|---|
|
IUPAC name
3,4′,5,7-Tetrahydroxyflavylium
| |
|
Systematic IUPAC name
3,5,7-Trihydroxy-2-(4-hydroxyphenyl)-1λ4-benzopyran-4-ylium | |
| Identifiers | |
3D model (
JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem
CID
|
|
| UNII | |
| |
| |
| Properties | |
| C15H11O5+ | |
| Molar mass | 271.24 g/mol |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Pelargonidin is an anthocyanidin, a type of plant pigment producing a characteristic orange color used in food and industrial dyes. [1]
Natural occurrences
Presence in flowers
Pelargonidin can be found in red geraniums (Geraniaceae). It is the predominant pigment causing the red coloration in the spathes of Philodendron (Araceae). The orange-coloured flowers of blue pimpernel ( Anagallis monelli, Myrsinaceae) have a higher concentration of pelargonidin pigment. Red and Pink Roses (Rosa) obtain their color from this phytochemical. [2]
Presence in food
Pelargonidin can be found in berries such as ripe raspberries and strawberries, as well as blueberries, blackberries, cranberries but also in saskatoon berries [3] and chokeberries. It is also found in plums and pomegranates. Pelargonidin gives red radishes their color. [4]
It is present in large amounts in kidney beans. [5]
Glycosides
In many plant systems, Pelargonidin can be added to a glucose molecule to form Pelargonidin 3-glucoside (callistephin). This is done by the 3GT, anthocyanin 3-O-glucosyltransferase gene. [6]

However this glucosidation reduces its antioxidant activity, [7] and changes the wavelength of max light absorbance from 520 nm to 516 nm. [8]
Acylated pelargonidin glycosides can be found in red-purple flowers of Ipomoea purpurea. [9]
See also
References
- ^ US granted 6,767,999, Smirnov, Vitaly; Sidorov, Viktor; Smirnova, Valentina, "Anthocyantin coloring agent and method for the production thereof from organic matter", published Nov 01, 2001, issued July 27, 2004
- ^ Huihua, Wan; Chao, Yu; Yu, Han; Xuelian, Guo (2019). "Determination of Flavonoids and Carotenoids and Their Contributions to Various Colors of Rose Cultivars (Rosa spp.)". Frontiers in Plant Science. 10: 123. doi: 10.3389/fpls.2019.00123. PMC 6379320. PMID 30809238.
- ^ Mazza, G. (2005). "Compositional and Functional Properties of Saskatoon Berry and Blueberry". International Journal of Fruit Science. 5 (3): 101–120. doi: 10.1300/J492v05n03_10. S2CID 85691882.
- ^ Takeshi Nishio (4 October 2017). Takeshi Nishio, Hiroyasu Kitashiba (ed.). The Radish Genome. Springer. p. 4. ISBN 978-3-319-59253-4.
- ^ Lin, Long-Ze; Harnly, James M.; Pastor-Corrales, Marcial S.; Luthria, Devanand L. (2008). "The polyphenolic profiles of common bean (Phaseolus vulgaris L.)". Food Chemistry. 107 (1): 399–410. doi: 10.1016/j.foodchem.2007.08.038. PMC 4276374. PMID 25544796.
- ^ Levisson, Mark; Patinios, Constantinios; Hein, Sascha; de Groot, Phillip A. (2018). "Engineering de novo anthocyanin production in Saccharomyces cerevisiae". Microbial Cell Factories. 17 (103): 103. doi: 10.1186/s12934-018-0951-6. PMC 6029064. PMID 29970082.
- ^ Li, Wenfeng; Gu, Mengyuan; Gong, Pengling; Wang, Jinxia (2021). "Glycosides changed the stability and antioxidant activity of pelargonidin". Lebensmittel-Wissenschaft & Technologie. 147 (3): 111581. doi: 10.1016/j.lwt.2021.111581. S2CID 235531625.
- ^ Gould, Kevin S. (2009). Anthocyanidins: Biosynthesis, Functions, and Applications. New York: Springer. p. 286. ISBN 978-0-387-77334-6.
- ^ Saito, N; Tatsuzawa, F; Yokoi, M; Kasahara, K; Iida, S; Shigihara, A; Honda, T (1996). "Acylated pelargonidin glycosides in red-purple flowers of Ipomoea purpurea". Phytochemistry. 43 (6): 1365–70. doi: 10.1016/s0031-9422(96)00501-8. PMID 8987912.
External links