| Plasmodium RESA, N-terminal | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| Symbol | PRESAN | ||||||||
| Pfam | PF09687 | ||||||||
| InterPro | IPR019111 | ||||||||
| CATH | 4jle | ||||||||
| |||||||||
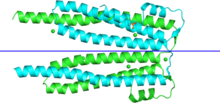
| Also IPR006526. The structure is chain-swapped: each side of the blue line is a "solution" monomer. | |||||||||
The Plasmodium helical interspersed subtelomeric proteins (PHIST) or ring-infected erythrocyte surface antigens (RESA) are a family of protein domains found in the malaria-causing Plasmodium species. It was initially identified as a short four-helical conserved region in the single-domain export proteins, [1] but the identification of this part associated with a DnaJ domain in P. falciparum RESA (named after the ring stage of the parasite) has led to its reclassification as the RESA N-terminal domain. This domain has been classified into three subfamilies, PHISTa, PHISTb, and PHISTc. [2]
The PHIST proteins are exported to the cytoplasm of the infected erythrocyte. The human malaria parasites P. falciparum and P. vivax have shown a lineage-specific expansion of proteins with this domain. [1] Of the two PHIST genes in the mouse parasite P. berghei, only one is required for infection. [3] The PHIST domain folds into three long helices (forming a bundle) and two smaller N-terminal helices, and is monomeric in solution. It binds PfEMP1 ATS C-terminus and plays a role in "knob" formation. [4]
| Ring-infected erythrocyte surface antigen | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Organism | |||||||
| Symbol | RESA | ||||||
| Alt. symbols | Pf155, RESA-1 | ||||||
| UniProt | P13830 | ||||||
| |||||||
The full RESA protein in P. falciparum also contains a few other domains, namely the DnaJ domain and the DnaJ-associated X domain. A part of the X-domain, RESA/P13830663-670, appears to bind and reinforce spectrin cytoskeleton so that each erythrocyte only hosts one parasite. [5]
P. falciparum isolate 3D7 encodes three RESA-family proteins, RESA-1 (P13830/
- ^ a b Sargeant TJ, Marti M, Caler E, Carlton JM, Simpson K, Speed TP, Cowman AF (2006). "Lineage-specific expansion of proteins exported to erythrocytes in malaria parasites". Genome Biology. 7 (2): R12. doi: 10.1186/gb-2006-7-2-r12. PMC 1431722. PMID 16507167.
- ^ Oakley MS, Kumar S, Anantharaman V, Zheng H, Mahajan B, Haynes JD, et al. (April 2007). "Molecular factors and biochemical pathways induced by febrile temperature in intraerythrocytic Plasmodium falciparum parasites". Infection and Immunity. 75 (4): 2012–25. doi: 10.1128/IAI.01236-06. PMC 1865691. PMID 17283083.
- ^ Moreira CK, Naissant B, Coppi A, Bennett BL, Aime E, Franke-Fayard B, et al. (29 March 2016). "The Plasmodium PHIST and RESA-Like Protein Families of Human and Rodent Malaria Parasites". PLOS ONE. 11 (3): e0152510. Bibcode: 2016PLoSO..1152510M. doi: 10.1371/journal.pone.0152510. PMC 4811531. PMID 27022937.
- ^ Oberli A, Slater LM, Cutts E, Brand F, Mundwiler-Pachlatko E, Rusch S, et al. (October 2014). "A Plasmodium falciparum PHIST protein binds the virulence factor PfEMP1 and comigrates to knobs on the host cell surface". FASEB Journal. 28 (10): 4420–33. doi: 10.1096/fj.14-256057. PMC 4202109. PMID 24983468.
- ^ Pei X, Guo X, Coppel R, Bhattacharjee S, Haldar K, Gratzer W, et al. (August 2007). "The ring-infected erythrocyte surface antigen (RESA) of Plasmodium falciparum stabilizes spectrin tetramers and suppresses further invasion". Blood. 110 (3): 1036–42. doi: 10.1182/blood-2007-02-076919. PMC 1924765. PMID 17468340.
- ^ Badaut C, Guyonnet L, Milet J, Renard E, Durand R, Viwami F, et al. (July 2015). "Immunoglobulin response to Plasmodium falciparum RESA proteins in uncomplicated and severe malaria". Malaria Journal. 14: 278. doi: 10.1186/s12936-015-0799-8. PMC 4502540. PMID 26178656.
- ^ Durand R, Migot-Nabias F, Andriantsoanirina V, Seringe E, Viwami F, Sagbo G, et al. (April 2012). "Possible association of the Plasmodium falciparum T1526C resa2 gene mutation with severe malaria". Malaria Journal. 11 (1): 128. doi: 10.1186/1475-2875-11-128. PMC 3422168. PMID 22533816.
| Plasmodium RESA, N-terminal | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| Symbol | PRESAN | ||||||||
| Pfam | PF09687 | ||||||||
| InterPro | IPR019111 | ||||||||
| CATH | 4jle | ||||||||
| |||||||||
| Also IPR006526. The structure is chain-swapped: each side of the blue line is a "solution" monomer. | |||||||||
The Plasmodium helical interspersed subtelomeric proteins (PHIST) or ring-infected erythrocyte surface antigens (RESA) are a family of protein domains found in the malaria-causing Plasmodium species. It was initially identified as a short four-helical conserved region in the single-domain export proteins, [1] but the identification of this part associated with a DnaJ domain in P. falciparum RESA (named after the ring stage of the parasite) has led to its reclassification as the RESA N-terminal domain. This domain has been classified into three subfamilies, PHISTa, PHISTb, and PHISTc. [2]
The PHIST proteins are exported to the cytoplasm of the infected erythrocyte. The human malaria parasites P. falciparum and P. vivax have shown a lineage-specific expansion of proteins with this domain. [1] Of the two PHIST genes in the mouse parasite P. berghei, only one is required for infection. [3] The PHIST domain folds into three long helices (forming a bundle) and two smaller N-terminal helices, and is monomeric in solution. It binds PfEMP1 ATS C-terminus and plays a role in "knob" formation. [4]
| Ring-infected erythrocyte surface antigen | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Organism | |||||||
| Symbol | RESA | ||||||
| Alt. symbols | Pf155, RESA-1 | ||||||
| UniProt | P13830 | ||||||
| |||||||
The full RESA protein in P. falciparum also contains a few other domains, namely the DnaJ domain and the DnaJ-associated X domain. A part of the X-domain, RESA/P13830663-670, appears to bind and reinforce spectrin cytoskeleton so that each erythrocyte only hosts one parasite. [5]
P. falciparum isolate 3D7 encodes three RESA-family proteins, RESA-1 (P13830/
- ^ a b Sargeant TJ, Marti M, Caler E, Carlton JM, Simpson K, Speed TP, Cowman AF (2006). "Lineage-specific expansion of proteins exported to erythrocytes in malaria parasites". Genome Biology. 7 (2): R12. doi: 10.1186/gb-2006-7-2-r12. PMC 1431722. PMID 16507167.
- ^ Oakley MS, Kumar S, Anantharaman V, Zheng H, Mahajan B, Haynes JD, et al. (April 2007). "Molecular factors and biochemical pathways induced by febrile temperature in intraerythrocytic Plasmodium falciparum parasites". Infection and Immunity. 75 (4): 2012–25. doi: 10.1128/IAI.01236-06. PMC 1865691. PMID 17283083.
- ^ Moreira CK, Naissant B, Coppi A, Bennett BL, Aime E, Franke-Fayard B, et al. (29 March 2016). "The Plasmodium PHIST and RESA-Like Protein Families of Human and Rodent Malaria Parasites". PLOS ONE. 11 (3): e0152510. Bibcode: 2016PLoSO..1152510M. doi: 10.1371/journal.pone.0152510. PMC 4811531. PMID 27022937.
- ^ Oberli A, Slater LM, Cutts E, Brand F, Mundwiler-Pachlatko E, Rusch S, et al. (October 2014). "A Plasmodium falciparum PHIST protein binds the virulence factor PfEMP1 and comigrates to knobs on the host cell surface". FASEB Journal. 28 (10): 4420–33. doi: 10.1096/fj.14-256057. PMC 4202109. PMID 24983468.
- ^ Pei X, Guo X, Coppel R, Bhattacharjee S, Haldar K, Gratzer W, et al. (August 2007). "The ring-infected erythrocyte surface antigen (RESA) of Plasmodium falciparum stabilizes spectrin tetramers and suppresses further invasion". Blood. 110 (3): 1036–42. doi: 10.1182/blood-2007-02-076919. PMC 1924765. PMID 17468340.
- ^ Badaut C, Guyonnet L, Milet J, Renard E, Durand R, Viwami F, et al. (July 2015). "Immunoglobulin response to Plasmodium falciparum RESA proteins in uncomplicated and severe malaria". Malaria Journal. 14: 278. doi: 10.1186/s12936-015-0799-8. PMC 4502540. PMID 26178656.
- ^ Durand R, Migot-Nabias F, Andriantsoanirina V, Seringe E, Viwami F, Sagbo G, et al. (April 2012). "Possible association of the Plasmodium falciparum T1526C resa2 gene mutation with severe malaria". Malaria Journal. 11 (1): 128. doi: 10.1186/1475-2875-11-128. PMC 3422168. PMID 22533816.